45 Cfr 46 Raised To Regulatory Status
Breaking News Today
Mar 12, 2025 · 6 min read
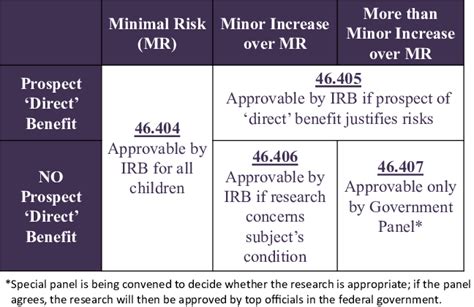
Table of Contents
45 CFR 46 Raised to Regulatory Status: A Comprehensive Overview
The elevation of 45 CFR 46, the Common Rule for the Protection of Human Subjects in Research, to regulatory status marks a significant shift in the landscape of human subjects research in the United States. This comprehensive guide delves into the implications of this change, exploring its historical context, key provisions, impact on researchers, and the future of human subjects protection.
Understanding the Common Rule (45 CFR 46)
45 CFR 46, officially titled "Protection of Human Subjects in Research," is a set of federal regulations that govern the ethical conduct of research involving human participants. It's a cornerstone of ethical research practices, aiming to protect the rights and welfare of individuals participating in studies. The rule applies to research conducted or supported by various federal agencies, including the National Institutes of Health (NIH), the Food and Drug Administration (FDA), and the Department of Defense (DOD).
Prior to its elevation to full regulatory status, 45 CFR 46 held a somewhat ambiguous position. While widely accepted and followed, its enforcement varied across different agencies and institutions. This lack of uniform enforcement presented challenges for researchers navigating the complex ethical considerations of human subject research.
Key Provisions of 45 CFR 46
The Common Rule outlines several crucial provisions designed to safeguard the rights and well-being of research participants. These include:
-
Informed Consent: This is arguably the most important aspect. Participants must be fully informed about the research's purpose, procedures, risks, and benefits before agreeing to participate. The informed consent process must be transparent and understandable, ensuring participants can make truly voluntary decisions.
-
IRB Review: All research covered by the Common Rule must undergo review by an Institutional Review Board (IRB). IRBs are independent committees that evaluate research proposals to ensure they adhere to ethical standards and protect participants' rights. They assess risks and benefits, scrutinize informed consent procedures, and monitor the ongoing conduct of research.
-
Privacy and Confidentiality: The Common Rule mandates the protection of participants' privacy and confidentiality. Researchers must take appropriate steps to safeguard personal information and prevent unauthorized disclosure. This includes secure data storage, anonymization techniques, and adherence to data privacy regulations.
-
Vulnerable Populations: The rule provides special protections for vulnerable populations, such as children, pregnant women, prisoners, and individuals with cognitive impairments. These groups may require additional safeguards and more stringent IRB review.
-
Data Safety and Monitoring: The Common Rule emphasizes the importance of data safety and monitoring plans to ensure participant safety throughout the research process. These plans might include regular reviews of data, mechanisms for early termination if safety concerns arise, and procedures for reporting adverse events.
The Impact of Regulatory Status
The elevation of 45 CFR 46 to full regulatory status strengthens its enforcement and creates a more standardized approach to human subjects research across all participating federal agencies. This has several significant implications:
-
Increased Accountability: With regulatory status, enforcement becomes more robust. Agencies have clearer authority to investigate violations and impose penalties, ensuring researchers comply with ethical guidelines.
-
Improved Consistency: The standardized approach reduces discrepancies in interpretation and enforcement across different agencies and institutions. Researchers now have a more consistent framework to follow, reducing ambiguity and simplifying compliance procedures.
-
Enhanced Protection for Participants: The stronger enforcement mechanisms offer greater protection for human participants, minimizing risks and maximizing their welfare. This enhanced protection fosters public trust in research and encourages broader participation in studies.
-
Streamlined Processes: While some initial adjustments were necessary, the transition to full regulatory status could eventually lead to more streamlined review processes for researchers. This simplification could accelerate research progress without compromising ethical standards.
-
Greater Transparency: The clearer regulatory framework fosters greater transparency in research practices. This promotes accountability and improves the public's understanding of how human subjects research is conducted.
Challenges and Considerations
While the transition to regulatory status offers significant benefits, it also presents some challenges:
-
Compliance Costs: Meeting the requirements of the Common Rule can entail substantial costs for institutions and researchers, including IRB review fees, training, and administrative burden. This increased cost could affect smaller research institutions and individual investigators.
-
Balancing Research Progress with Ethical Protection: Striking a balance between rigorous ethical protection and the need for efficient research remains a critical challenge. Overly burdensome regulations could potentially stifle innovation and delay important research breakthroughs.
-
International Harmonization: The Common Rule primarily focuses on research conducted within the United States. Greater harmonization with international ethical guidelines and regulations is needed to facilitate global collaborations and ensure consistent ethical standards worldwide.
-
Technological Advancements: Rapid advancements in technology, such as artificial intelligence and big data, present new ethical considerations. The Common Rule needs to adapt to these advancements to adequately address the ethical challenges posed by emerging technologies.
The Future of Human Subjects Protection
The regulatory elevation of 45 CFR 46 represents a pivotal moment in the ongoing evolution of human subjects protection. Looking ahead, several areas warrant attention:
-
Ongoing Refinement of the Common Rule: Regular review and updates to the Common Rule are essential to ensure it remains relevant and effective in addressing emerging ethical challenges. This continuous adaptation will help maintain a balance between ethical protection and the advancement of scientific knowledge.
-
Focus on Education and Training: Comprehensive training and educational programs for researchers, IRB members, and other stakeholders are crucial to ensure widespread understanding and effective implementation of the Common Rule. This investment in education will foster a culture of ethical research and promote compliance.
-
Technological Advancements and Ethical Considerations: The integration of emerging technologies into research requires proactive ethical considerations and adaptations to the Common Rule. Addressing these challenges requires collaborative efforts between researchers, ethicists, policymakers, and technology developers.
-
Strengthening Collaboration and Communication: Enhanced collaboration and open communication among researchers, IRBs, regulatory agencies, and other stakeholders are necessary to address the complexities of human subjects research and ensure effective implementation of the Common Rule. This collaborative approach is essential for the continued improvement of ethical protections.
Conclusion
The elevation of 45 CFR 46 to regulatory status signifies a substantial step forward in protecting human participants in research. While challenges remain, the strengthened enforcement, increased consistency, and heightened accountability contribute significantly to improving research ethics. Ongoing efforts to refine the Common Rule, invest in education, and address emerging ethical challenges are crucial for maintaining a robust and adaptable framework that safeguards human rights and fosters responsible scientific advancement. The ultimate goal remains to ensure that human subjects research is conducted ethically, fostering trust and maximizing the benefits while minimizing the risks to participants. The future of ethical research hinges on the ongoing commitment to these principles and continuous adaptation to the ever-evolving landscape of scientific inquiry.
Latest Posts
Latest Posts
-
A Phrase Expressing The Aim Or Group Of A Party
May 09, 2025
-
Their Eyes Were Watching God Important Quotes
May 09, 2025
-
Which Form Of Energy Does An Endothermic Reaction Use
May 09, 2025
-
Commercial Refrigeration Equipment For Eggs Produce And Dairy Uses
May 09, 2025
-
Why Should You Work To Be An Informed Consumer
May 09, 2025
Related Post
Thank you for visiting our website which covers about 45 Cfr 46 Raised To Regulatory Status . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.