A Catalyst Increases The Rate Of A Chemical Reaction By
Breaking News Today
Mar 20, 2025 · 5 min read
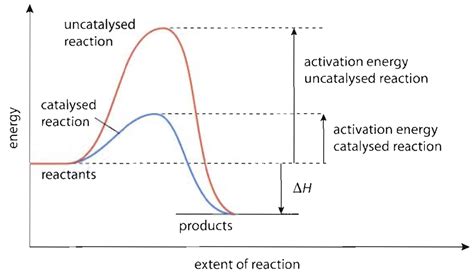
Table of Contents
A Catalyst Increases the Rate of a Chemical Reaction By… Lowering the Activation Energy
Chemical reactions are the foundation of all processes in the universe, from the simplest biological functions to the most complex industrial processes. The speed at which these reactions occur, however, can vary dramatically. This is where catalysts come in – substances that dramatically increase the rate of a chemical reaction without being consumed in the process. But how do they achieve this remarkable feat? The answer lies in their ability to lower the activation energy of the reaction.
Understanding Activation Energy: The Energy Barrier to Reaction
Before diving into the role of catalysts, we need to grasp the concept of activation energy (Ea). Imagine a chemical reaction as a ball rolling down a hill. The hill represents the energy barrier the reactants must overcome to transform into products. This barrier is the activation energy. The higher the hill (higher Ea), the slower the reaction, as fewer reactant molecules possess the necessary energy to climb the hill and initiate the reaction. Conversely, a lower hill (lower Ea) means a faster reaction.
The Role of Molecular Collisions and Energy Distribution
Chemical reactions occur when reactant molecules collide with sufficient energy and the correct orientation. The collision theory explains this. Not all collisions are productive; only those with enough energy to surpass the activation energy lead to a reaction. The distribution of kinetic energy within a collection of molecules follows a Boltzmann distribution. This means that only a fraction of molecules possess the necessary activation energy at any given temperature.
How Catalysts Lower the Activation Energy
Catalysts accelerate reactions by providing an alternative reaction pathway with a lower activation energy. Instead of the reactants needing to overcome the high-energy barrier of the uncatalyzed reaction, the catalyst facilitates a reaction pathway with a lower energy barrier, hence speeding up the reaction.
Different Mechanisms of Catalysis:
Catalysts achieve this lowering of activation energy through various mechanisms, depending on the type of catalyst and the reaction:
-
Providing an alternative reaction mechanism: Catalysts often interact with reactants, forming intermediate complexes. These complexes then react more easily, leading to the formation of products and the regeneration of the catalyst. This new mechanism has a lower activation energy than the uncatalyzed reaction.
-
Stabilizing the transition state: The transition state is a high-energy intermediate structure formed during the reaction. Catalysts can stabilize this transition state, reducing the energy required to reach it and therefore lowering the activation energy.
-
Increasing the frequency of effective collisions: While primarily focused on lowering the energy barrier, some catalysts also increase the frequency of collisions between reactants with proper orientation, contributing to the increased reaction rate.
-
Surface Catalysis: Heterogeneous catalysts, which are in a different phase than the reactants (e.g., solid catalyst in a liquid or gaseous reaction), often work by providing a large surface area for reactant molecules to adsorb onto. This increases the concentration of reactants at the catalytic surface and facilitates the reaction.
Examples of Catalytic Mechanisms:
Consider the decomposition of hydrogen peroxide (H₂O₂):
2H₂O₂ → 2H₂O + O₂
This reaction is slow, but manganese dioxide (MnO₂) catalyzes it effectively. The MnO₂ provides a surface where H₂O₂ molecules can adsorb and decompose into water and oxygen. The catalyst participates in the reaction but is not consumed.
Another example is the Haber-Bosch process for ammonia synthesis:
N₂ + 3H₂ ↔ 2NH₃
This crucial industrial process relies on an iron catalyst to significantly accelerate the reaction. The iron catalyst facilitates the breaking and forming of bonds involved in the conversion of nitrogen and hydrogen to ammonia.
Types of Catalysts and Their Applications:
Catalysts are broadly classified into several types:
-
Homogeneous Catalysts: These catalysts are in the same phase as the reactants (e.g., liquid catalyst in a liquid reaction). They often form intermediate complexes with the reactants, facilitating the reaction. Examples include many transition metal complexes used in organic synthesis.
-
Heterogeneous Catalysts: These catalysts are in a different phase than the reactants (e.g., solid catalyst in a liquid or gas reaction). They typically operate by providing a surface for the reactants to adsorb and react. Examples include zeolites used in petroleum refining and platinum used in catalytic converters.
-
Enzyme Catalysts (Biocatalysts): Enzymes are biological catalysts that accelerate biochemical reactions. They exhibit remarkable specificity and efficiency due to their intricate three-dimensional structures. Their mechanisms often involve specific binding sites for substrates and the formation of enzyme-substrate complexes.
Applications across Industries:
Catalysts are indispensable across various industrial sectors:
-
Petroleum refining: Catalysts are essential for cracking, reforming, and isomerization processes in oil refineries, producing valuable fuels and petrochemicals.
-
Chemical synthesis: Catalysts are crucial for synthesizing countless chemicals, from plastics and pharmaceuticals to fertilizers and agricultural chemicals.
-
Automotive industry: Catalytic converters in automobiles use catalysts to convert harmful pollutants in exhaust gases into less harmful substances.
-
Environmental remediation: Catalysts play a vital role in cleaning up pollutants, including air and water purification processes.
Factors Affecting Catalytic Activity:
Several factors influence the effectiveness of catalysts:
-
Temperature: While catalysts lower the activation energy, increasing the temperature generally enhances the reaction rate by increasing the number of molecules with sufficient energy to overcome the lowered activation energy barrier. However, excessively high temperatures can deactivate some catalysts.
-
Surface area (for heterogeneous catalysts): A larger surface area provides more sites for reactant adsorption and reaction, increasing catalytic activity.
-
Catalyst concentration (for homogeneous catalysts): Increasing catalyst concentration generally increases the reaction rate, but the effect may level off at high concentrations.
-
Presence of inhibitors or poisons: Certain substances can bind to the active sites of a catalyst, preventing its interaction with reactants and reducing catalytic activity. These are known as catalyst poisons.
-
Catalyst support: The material supporting the catalyst (especially in heterogeneous catalysis) can significantly influence its activity and stability.
Conclusion: The Indispensable Role of Catalysts
Catalysts are essential for countless chemical processes, both natural and industrial. Their ability to dramatically increase reaction rates by lowering the activation energy has revolutionized various industries. Understanding the fundamental mechanisms of catalysis, the different types of catalysts, and the factors influencing their activity is crucial for designing and optimizing catalytic processes for a sustainable future. Further research continues to explore novel catalyst materials and designs to improve the efficiency and sustainability of chemical processes, addressing pressing global challenges in energy, environment, and materials science. The exploration of novel catalysts and the deeper understanding of the underlying mechanisms remain active areas of research and technological development. The field of catalysis is dynamic and constantly evolving, promising further advancements in the years to come.
Latest Posts
Latest Posts
-
While Revising An Argumentative Essay A Writer Should
Mar 20, 2025
-
In Addition To Posts From Friends And Paid For Advertisements
Mar 20, 2025
-
Letrs Unit 1 Session 7 Check For Understanding
Mar 20, 2025
-
What Should Be Prioritized When Creating A Budget Everfi
Mar 20, 2025
-
Nihss Certification Nihss Answer Key Group B
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about A Catalyst Increases The Rate Of A Chemical Reaction By . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
