A Particle-level Diagram Of A Metallic Element
Breaking News Today
Mar 25, 2025 · 7 min read
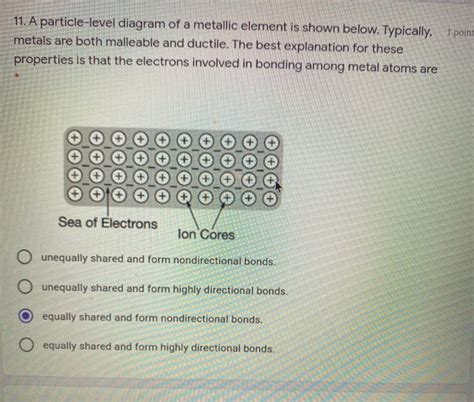
Table of Contents
A Particle-Level Diagram of a Metallic Element: Delving into the Microscopic World
Understanding the properties of metals requires a deep dive into their atomic structure. This article explores the particle-level diagram of a metallic element, explaining the arrangement of atoms, electrons, and the forces that govern their behavior. We'll examine the implications of this structure for the characteristic metallic properties like conductivity, malleability, and ductility.
The Sea of Electrons: A Defining Feature
Unlike ionic or covalent compounds, metallic elements exhibit a unique bonding arrangement. Instead of forming discrete molecules with localized electrons, metal atoms are bonded through a sea of delocalized electrons. This "sea" is a crucial aspect of understanding their macroscopic properties.
Delocalized Electrons: The Key Players
Imagine a lattice of positively charged metal ions (cations) arranged in a highly ordered, three-dimensional structure. These ions are not rigidly bound to each other, but rather held together by the electrostatic attraction to the surrounding cloud of delocalized electrons. These electrons are not associated with any particular atom; instead, they are free to move throughout the entire metallic structure. This mobility is the foundation of many metallic properties.
Visualizing the Sea: A Particle-Level Diagram
A simplified particle-level diagram might show:
- Positive Ions: Represented as circles with "+" symbols, arranged in a regular, repeating pattern. This pattern reflects the crystal lattice structure specific to the metal (e.g., body-centered cubic, face-centered cubic, hexagonal close-packed). The size of the circle can represent the relative size of the ion.
- Electron Cloud: Represented as a diffuse cloud of dots or a shaded region surrounding the positive ions. The density of the dots or shading would indicate the electron concentration, which can vary depending on the metal and its condition.
Example: Consider sodium (Na). A particle-level diagram of sodium metal would show sodium ions (Na⁺) arranged in a body-centered cubic lattice, surrounded by a sea of delocalized valence electrons.
The Influence of Atomic Structure on Metallic Properties
The "sea of electrons" model effectively explains several key properties of metals:
1. Electrical Conductivity:
The ease with which metals conduct electricity stems directly from the delocalized electrons. When an electric field is applied, these freely moving electrons can readily migrate through the metal, carrying the charge and establishing an electric current. This explains why metals are excellent conductors of electricity, unlike most non-metals.
2. Thermal Conductivity:
Similarly, the mobile electrons facilitate efficient heat transfer. When one part of a metal is heated, the kinetic energy of the electrons in that region increases. These energetic electrons quickly collide with neighboring electrons and ions, transferring energy throughout the material. This explains why metals are typically good conductors of heat.
3. Malleability and Ductility:
The ability of metals to be hammered into thin sheets (malleability) and drawn into wires (ductility) is linked to the non-directional nature of metallic bonding. When a metallic object is deformed, the layers of metal ions can slide past each other relatively easily. The "sea" of electrons acts as a lubricant, preventing the positive ions from repelling each other strongly and causing the material to fracture. This contrasts sharply with the behavior of ionic solids, where the strong directional bonds make them brittle.
4. Luster (Metallic Sheen):
The characteristic shine or luster of metals is attributed to the interaction of light with the delocalized electrons. When light strikes the metal's surface, the electrons absorb and then re-emit the light, resulting in the reflective surface we observe. The specific color of the luster can depend on the metal's electronic structure and its interactions with different wavelengths of light.
5. High Melting and Boiling Points:
The strong electrostatic attraction between the positively charged metal ions and the sea of electrons requires a significant amount of energy to overcome. This leads to relatively high melting and boiling points for most metals, although there's considerable variation depending on factors like the number of delocalized electrons and the size of the metal ions.
Variations in Metallic Structures: Crystal Lattices
The arrangement of metal ions in the lattice is not uniform across all metals. Different metals adopt different crystal structures, including:
1. Body-Centered Cubic (BCC):
Each atom has eight nearest neighbors and six next-nearest neighbors. Examples include iron (Fe), chromium (Cr), and tungsten (W). A particle-level diagram for a BCC structure would show a cube with an atom at each corner and one in the center.
2. Face-Centered Cubic (FCC):
Each atom has twelve nearest neighbors. Examples include copper (Cu), aluminum (Al), and gold (Au). An FCC diagram would show a cube with atoms at each corner and in the center of each face.
3. Hexagonal Close-Packed (HCP):
Each atom has twelve nearest neighbors, but the arrangement differs from FCC. Examples include magnesium (Mg), zinc (Zn), and titanium (Ti). The HCP structure is more complex to visualize than BCC or FCC.
Beyond the Simplified Model: Alloying and Imperfections
The "sea of electrons" model provides a fundamental understanding, but real metals are more complex. Alloying (mixing different metals) significantly alters properties. The introduction of impurity atoms can disrupt the perfect crystal lattice, creating imperfections like vacancies (missing atoms) and dislocations (disruptions in the lattice structure). These imperfections influence the mechanical strength, electrical conductivity, and other properties.
Alloying: Modifying Properties
Combining different metals results in alloys with properties often superior to those of the constituent metals. For example, steel (an alloy of iron and carbon) is much stronger than pure iron. The added carbon atoms disrupt the iron lattice, inhibiting the easy slippage of ion layers and thereby increasing strength and hardness. Particle-level diagrams of alloys become more intricate, showing a mixture of different metal ions within the lattice structure.
Crystal Defects: Influencing Behavior
Crystal defects are unavoidable in real materials. Vacancies, interstitial atoms (atoms squeezed into spaces between lattice sites), and dislocations all impact material properties. Dislocations, for instance, can make a material more ductile by providing pathways for plastic deformation. The study of these defects is a significant part of materials science and engineering.
Advanced Concepts: Band Theory and Fermi Level
For a more complete picture, one needs to delve into band theory. This quantum mechanical model describes the energy levels of electrons in a solid. In metals, the valence electrons occupy a partially filled band called the conduction band. This allows for easy electron movement and explains the excellent conductivity. The Fermi level represents the highest occupied energy level at absolute zero temperature.
Band Theory: A Deeper Dive into Electron Behavior
Band theory provides a more accurate description of electron behavior in metals than the simple "sea of electrons" model. It explains why some materials are conductors, insulators, or semiconductors based on the spacing and filling of energy bands. In metals, the overlapping of energy bands allows for free electron movement.
Fermi Level: A Key Concept in Metal Physics
The Fermi level plays a crucial role in determining a metal's electrical and thermal properties. It is the energy level at which the probability of an electron occupying a state is 0.5 at absolute zero. Understanding the Fermi level is essential for exploring more advanced aspects of metal physics, such as the behavior of metals at low temperatures and the effects of magnetic fields.
Conclusion: From Simple Diagrams to Complex Behavior
The particle-level diagram provides a crucial visual starting point for understanding the behavior of metallic elements. While the simple representation of positive ions surrounded by a sea of electrons captures the essence of metallic bonding, the reality is far richer and more complex. Factors like crystal structure, alloying, crystal defects, band theory, and Fermi level all contribute to the wide range of properties exhibited by metals, making them indispensable materials in countless applications. Further exploration of these advanced concepts provides a deeper appreciation for the fascinating microscopic world that governs the macroscopic behavior of metals.
Latest Posts
Latest Posts
-
Hay 13 6 Chicas Y 20 12 Chicos
Mar 27, 2025
-
Se Sugiere Buscar Una Casa En Un Barrio Seguro Safe
Mar 27, 2025
-
What Are The Key Clauses In Ap Government
Mar 27, 2025
-
Which Of The Following Best Describes An Inside Attacker
Mar 27, 2025
-
Post Test The Early Twentieth Century Modernism
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about A Particle-level Diagram Of A Metallic Element . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
