A State Function Is Best Described As
Breaking News Today
Mar 12, 2025 · 6 min read
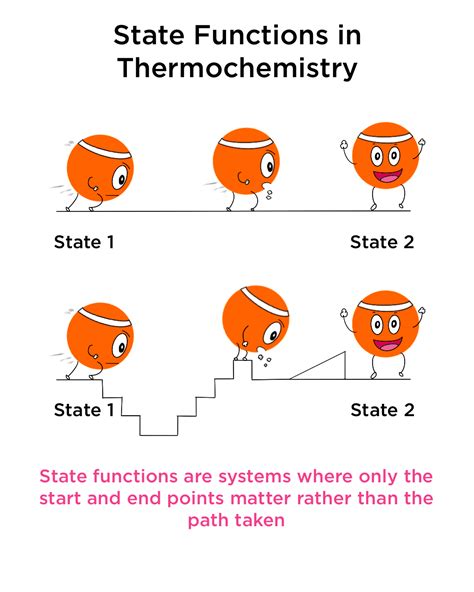
Table of Contents
A State Function is Best Described as... Path-Independent!
Understanding state functions is crucial in thermodynamics and various branches of chemistry and physics. Often shrouded in complex equations and abstract concepts, the core idea is surprisingly straightforward: a state function's value depends only on its current state, not the path taken to reach it. This seemingly simple distinction has profound implications in how we analyze and model systems. This comprehensive guide will delve deep into the definition, characteristics, and applications of state functions, illustrating their significance with real-world examples.
Defining a State Function: The Essence of Path Independence
A state function, also known as a point function, is a property of a thermodynamic system whose value depends solely on the current equilibrium state of the system. This means its value is independent of the process or pathway used to reach that state. Imagine climbing a mountain; your elevation (a state function) is determined solely by your current altitude, irrespective of the winding trail you took to get there. You could have taken a steep, rocky path, or a gentler, longer route – your final elevation remains the same.
This path independence is the defining characteristic of a state function. Conversely, quantities that depend on the pathway are called path functions. The work done in climbing the mountain is a path function; a longer, less steep path will require less work than a shorter, steeper one, even if the final altitude is the same.
Key Characteristics of State Functions
- Path Independence: This is the cornerstone characteristic. The change in a state function between two states is always the same, regardless of the path taken.
- Exact Differentials: State functions have exact differentials. This mathematical property reflects the path independence; the integral of an exact differential is path-independent.
- State Variables: They are defined by the state of the system. The state is typically defined by a set of parameters like temperature, pressure, volume, and internal energy. Knowing these parameters allows us to determine the value of a state function.
- Additivity: The value of a state function for a composite system is the sum of the values for its individual components.
Examples of State Functions: The Thermodynamic Big Five
Several fundamental properties in thermodynamics are state functions. These are often referred to as the "big five":
-
Internal Energy (U): Represents the total energy stored within a system. It includes kinetic energy (due to molecular motion) and potential energy (due to intermolecular forces). The change in internal energy (ΔU) is crucial in the first law of thermodynamics.
-
Enthalpy (H): Defined as H = U + PV, where P is pressure and V is volume. Enthalpy is a particularly useful state function for constant-pressure processes, as the change in enthalpy (ΔH) represents the heat absorbed or released under constant pressure. Many chemical reactions occur under constant atmospheric pressure, making enthalpy changes crucial for understanding reaction energetics.
-
Entropy (S): A measure of disorder or randomness within a system. The second law of thermodynamics states that the total entropy of an isolated system can only increase over time or remain constant in ideal cases where the system is in a steady state or undergoing a reversible process. The change in entropy (ΔS) is related to the spontaneity of a process.
-
Gibbs Free Energy (G): Defined as G = H - TS, where T is temperature. Gibbs free energy is a powerful state function used to determine the spontaneity of a process at constant temperature and pressure. A negative change in Gibbs free energy (ΔG) indicates a spontaneous process.
-
Helmholtz Free Energy (A): Defined as A = U - TS. Similar to Gibbs free energy, Helmholtz free energy predicts spontaneity, but at constant temperature and volume. It's particularly useful in systems with fixed volumes.
Contrasting State Functions with Path Functions: The Importance of the Distinction
To fully grasp the concept of a state function, it's essential to understand its contrast with path functions. A path function is a property whose value depends on the path taken between two states.
Examples of Path Functions:
-
Work (W): The work done on or by a system depends on the specific process. For example, the work done in compressing a gas isothermally is different from the work done adiabatically.
-
Heat (Q): Similar to work, the heat transferred to or from a system depends heavily on the path taken. Different processes result in different heat transfers, even if the initial and final states are the same.
-
Heat Capacity (C): Heat capacity varies depending on the conditions under which it is measured. For example, the heat capacity at constant volume (Cv) is different from the heat capacity at constant pressure (Cp). While heat capacity is itself not a state function, it is used in calculating changes in state functions such as enthalpy and internal energy
The difference between state and path functions has far-reaching consequences. For instance, when calculating the change in internal energy (ΔU), we can use any convenient path, as ΔU is independent of the path. However, calculating work (W) or heat (Q) requires specifying the exact path the system follows.
Applications of State Functions: From Chemistry to Engineering
The importance of state functions extends far beyond theoretical thermodynamics. Their application spans numerous fields:
Chemistry:
-
Reaction Enthalpy (ΔHrxn): Calculating the heat released or absorbed during a chemical reaction is crucial for designing and optimizing chemical processes. Since enthalpy is a state function, we can use various methods (e.g., calorimetry, Hess's Law) to determine ΔHrxn.
-
Reaction Entropy (ΔSrxn): Predicting the spontaneity of a reaction often involves determining the change in entropy. State functions provide a framework for understanding and predicting reaction spontaneity.
-
Equilibrium Constants: Equilibrium constants, which dictate the position of equilibrium in a reversible reaction, are related to changes in Gibbs free energy, a state function.
Engineering:
-
Power Cycles: Designing efficient power cycles (e.g., Carnot cycle, Rankine cycle) relies heavily on understanding state functions like internal energy, enthalpy, and entropy. These cycles operate by manipulating state functions to extract work from heat.
-
Refrigeration Cycles: Similarly, refrigeration cycles utilize state functions to transfer heat from a low-temperature reservoir to a high-temperature reservoir.
-
Chemical Process Design: Optimizing chemical processes, such as distillation, requires understanding the energy changes involved, which are directly related to state functions.
Advanced Concepts and Considerations
The concept of state functions can be further explored through more advanced topics:
-
Maxwell Relations: These relationships, derived from the exact differentials of state functions, provide valuable connections between different thermodynamic properties.
-
Partial Derivatives: Understanding partial derivatives is essential for manipulating state function relationships and calculating changes in state functions under various conditions.
-
Thermodynamic Potentials: Beyond enthalpy and Gibbs free energy, other thermodynamic potentials, like Helmholtz free energy and grand potential, are useful in specific scenarios.
-
Statistical Mechanics: State functions can be derived and explained from a microscopic perspective through statistical mechanics. This connects the macroscopic properties we observe to the underlying behavior of individual molecules.
Conclusion: The Fundamental Role of State Functions
In conclusion, understanding state functions is fundamental to mastering thermodynamics and its applications. Their path independence simplifies the analysis of complex systems, allowing us to focus on the initial and final states rather than the intricate details of the process. Whether calculating the energy changes in a chemical reaction or designing an efficient power cycle, the concept of state functions is a cornerstone of scientific understanding and technological advancement. The ability to determine and predict changes in these properties through various experimental and computational methods remains crucial for further advancements across diverse fields. By understanding the path independence inherent in state functions, we gain a powerful tool for analyzing and manipulating physical systems.
Latest Posts
Latest Posts
-
What Is Meant By The Unexpected Consequences Of Environmental Manipulation
Mar 12, 2025
-
Are You Smarter Than A Third Grader Quiz
Mar 12, 2025
-
How Has Ocean Exploration Increased Human Understanding Of Aquatic Ecosystems
Mar 12, 2025
-
Some Enlightenment Thinkers Were Afraid Of This
Mar 12, 2025
-
Which Sentence From The Passage Best Supports His Inference
Mar 12, 2025
Related Post
Thank you for visiting our website which covers about A State Function Is Best Described As . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
