All Organic Compounds Contain: Question 7 Options: Oxygen Hydrogen Carbon
Breaking News Today
Mar 25, 2025 · 6 min read
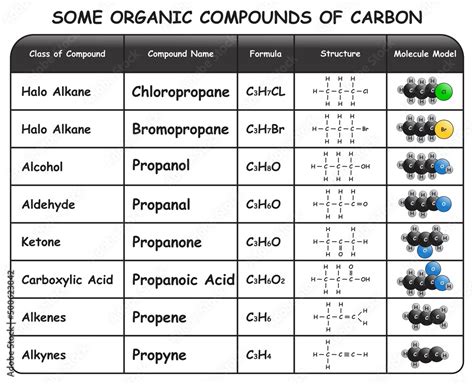
Table of Contents
All Organic Compounds Contain: Carbon – A Deep Dive into Organic Chemistry
The question, "All organic compounds contain: oxygen, hydrogen, carbon," presents a fundamental concept in organic chemistry. While oxygen and hydrogen are frequently found in organic compounds, the definitive answer is carbon. This article delves into the reasons why carbon is the cornerstone of organic chemistry, exploring its unique properties and the vast diversity of organic molecules it forms.
The Unique Properties of Carbon
Carbon's central role in organic chemistry stems from its exceptional ability to form stable covalent bonds. Unlike many other elements, carbon exhibits tetravalency, meaning it can form four covalent bonds with other atoms. This capacity allows for the creation of an incredibly vast array of structures, from simple linear chains to complex branched and ringed molecules.
1. Catenaion: The Power of Carbon-Carbon Bonds
Carbon's most remarkable property is its ability to bond extensively with itself, a phenomenon known as catenaion. This self-linking capability allows carbon atoms to form long chains, branched structures, and rings, laying the foundation for the incredible diversity observed in organic molecules. This contrasts sharply with other elements, which may form limited chains or exhibit significantly less structural variety.
2. Covalent Bonding: The Foundation of Organic Molecules
Carbon forms strong covalent bonds with other carbon atoms and a wide range of other elements, including hydrogen, oxygen, nitrogen, sulfur, and halogens. These covalent bonds involve the sharing of electrons between atoms, creating stable and relatively unreactive molecules under normal conditions. The strength and stability of these bonds contribute to the resilience and complexity of organic compounds.
3. Isomerism: The Multiplicity of Organic Molecules
The ability of carbon to form multiple bonds (single, double, and triple bonds) and its capacity for catenaion lead to a phenomenon called isomerism. Isomers are molecules with the same chemical formula but different structural arrangements. This means that a single chemical formula can represent many different organic molecules with distinct properties and functions. For example, consider the formula C₄H₁₀: it represents two isomers, butane and isobutane, which have slightly different physical and chemical characteristics. This vast array of isomers significantly increases the structural diversity of organic compounds.
4. Functional Groups: The Building Blocks of Organic Reactivity
While carbon forms the backbone of organic molecules, the presence of specific groups of atoms, called functional groups, imparts characteristic chemical properties to the molecule. These functional groups, which often include oxygen, nitrogen, or sulfur atoms, determine how a molecule will react chemically. For example, the presence of a hydroxyl group (-OH) makes a molecule an alcohol, while a carboxyl group (-COOH) makes it a carboxylic acid. The vast array of functional groups further expands the chemical diversity within organic chemistry.
The Role of Oxygen and Hydrogen in Organic Compounds
While carbon is the essential element, oxygen and hydrogen are ubiquitous components of many organic molecules. Their presence significantly impacts the properties and functions of these compounds.
1. Oxygen: Diverse Roles in Organic Molecules
Oxygen frequently appears in various functional groups, influencing the molecule's reactivity and properties. For instance, alcohols (-OH), ketones (C=O), aldehydes (C=O), and carboxylic acids (-COOH) all incorporate oxygen atoms within their functional groups. Oxygen's electronegativity also plays a vital role in determining the polarity and reactivity of these functional groups.
2. Hydrogen: Essential for Structure and Reactivity
Hydrogen is the most common element in organic compounds, often bonding directly to carbon atoms to form hydrocarbon chains. The presence of hydrogen atoms affects the molecule's shape, polarity, and reactivity. In many reactions, hydrogen atoms may be gained or lost, influencing the molecule's chemical behavior.
Examples of Organic Compounds and Their Structures
To illustrate the diversity and complexity of organic molecules built around carbon backbones, let's consider a few examples:
1. Methane (CH₄): The Simplest Organic Compound
Methane is the simplest organic molecule, containing a single carbon atom bonded to four hydrogen atoms in a tetrahedral arrangement. It's a gas at room temperature and is a significant component of natural gas.
2. Ethane (C₂H₆): A Simple Hydrocarbon Chain
Ethane comprises two carbon atoms bonded together, each with three hydrogen atoms attached. This simple chain demonstrates the fundamental concept of carbon-carbon bonding.
3. Ethanol (C₂H₅OH): An Alcohol with a Hydroxyl Group
Ethanol contains a hydroxyl group (-OH) attached to a two-carbon chain. The hydroxyl group imparts alcohol-specific properties, such as its ability to dissolve in water and its slightly polar nature.
4. Glucose (C₆H₁₂O₆): A Complex Carbohydrate
Glucose is a six-carbon sugar crucial for energy storage and metabolism in living organisms. Its structure contains multiple hydroxyl groups and a ring structure, highlighting the complexity that carbon’s bonding allows.
5. Proteins: Complex Polymers of Amino Acids
Proteins are large biomolecules formed from chains of amino acids. Each amino acid contains a carbon atom bonded to an amino group (-NH₂), a carboxyl group (-COOH), and a side chain, which determines the amino acid's unique properties. The vast number of possible amino acid sequences accounts for the incredible diversity of protein structures and functions.
6. DNA and RNA: Nucleic Acids Carrying Genetic Information
Deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) are crucial biomolecules responsible for carrying and transferring genetic information. These molecules are formed from nucleotide subunits, each of which contains a carbon-based sugar (deoxyribose in DNA, ribose in RNA), a phosphate group, and a nitrogenous base. The sequence of these bases in DNA and RNA determines the genetic code of an organism.
These examples highlight the astonishing diversity of organic compounds that arise from the unique properties of carbon. The ability of carbon to form strong covalent bonds, undergo catenaion, and form an incredibly vast array of isomers underpins the complexity and importance of organic chemistry.
Why Carbon? A Closer Look at Alternatives
While other elements can form chains and complex molecules, none match carbon's versatility. Silicon, for instance, is in the same group as carbon and can form chains, but these chains are far less stable and much more reactive than their carbon counterparts. Furthermore, silicon's ability to form multiple bonds is significantly limited, restricting the variety of structural forms it can create.
The stability of carbon-carbon bonds is crucial for the existence of large, complex organic molecules. The strength and relative inertness of these bonds allow for the formation of stable macromolecules, like proteins and DNA, essential for life as we know it.
Conclusion: Carbon – The Backbone of Life
The answer to "All organic compounds contain: oxygen, hydrogen, carbon" is unequivocally carbon. Carbon's unique properties, including its tetravalency and ability to form strong covalent bonds with itself and other elements, allow for the creation of a vast array of organic molecules with diverse structures and functions. While oxygen and hydrogen are common components of many organic compounds, they are not universally present, while carbon is fundamental to the definition and existence of this crucial branch of chemistry. The incredible diversity of organic molecules, from simple hydrocarbons to complex biomolecules, stands as a testament to the central role of carbon in chemistry and life itself. The study of organic chemistry continues to unveil the complexities and potential of these carbon-based molecules, promising breakthroughs in numerous fields, including medicine, materials science, and environmental science.
Latest Posts
Latest Posts
-
Which Of The Following Is Not A Characteristic Of Plants
Mar 28, 2025
-
A Patient With Spontaneous Respirations Is Breathing
Mar 28, 2025
-
Vicente Es De Costa Rica El Es De
Mar 28, 2025
-
Explain The Reciprocal Relationship Between Human Society And Limiting Factors
Mar 28, 2025
-
The Organization Of Beats Into Regular Groupings Is Called
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about All Organic Compounds Contain: Question 7 Options: Oxygen Hydrogen Carbon . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
