All Three Pathways For Complement Activation Quizlet
Breaking News Today
Mar 31, 2025 · 6 min read
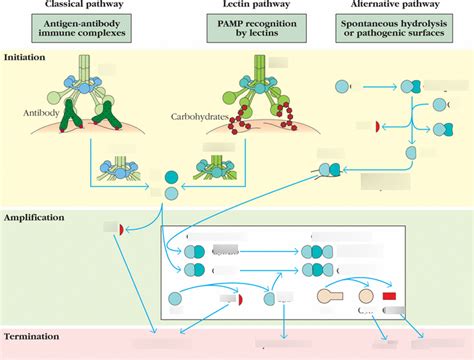
Table of Contents
All Three Pathways for Complement Activation: A Deep Dive
The complement system is a crucial part of the innate immune system, acting as a bridge between innate and adaptive immunity. Its primary function is to eliminate pathogens and damaged cells. This intricate system achieves this through a cascade of enzymatic reactions, initiated via three distinct pathways: the classical, lectin, and alternative pathways. While they differ in their initiators, all three pathways converge at the central point of C3 convertase formation, leading to the same effector functions. Understanding these pathways is fundamental to grasping the complexities of the immune response. This comprehensive guide will delve into each pathway, highlighting their similarities and differences, and addressing key concepts often tested in examinations.
The Classical Pathway: Antibody-Mediated Activation
The classical pathway, the first complement pathway discovered, is antibody-dependent, meaning it requires the presence of antibodies bound to an antigen. This pathway is a powerful component of the adaptive immune response, effectively targeting specific pathogens already marked by antibodies.
Initiation: Antigen-Antibody Complexes
The process starts when antibodies (primarily IgM and IgG isotypes) bind to specific antigens on the surface of a pathogen or other target cell. This binding exposes a previously hidden region on the Fc portion of the antibody, creating a binding site for the first complement protein, C1q.
C1 Complex Formation and Activation:
C1q, a large hexameric protein, binds to the antibody's Fc region. This binding triggers a conformational change in C1q, activating the associated serine proteases C1r and C1s. C1r autoactivates, then activates C1s, initiating the enzymatic cascade.
C4 and C2 Cleavage:
Activated C1s cleaves C4 into C4a and C4b. C4b binds covalently to the pathogen's surface. Subsequently, C1s cleaves C2 into C2a and C2b. C2a binds to the surface-bound C4b, forming the classical C3 convertase (C4b2a).
C3 Convertase Activity and Amplification:
The C3 convertase (C4b2a) is a crucial enzyme, cleaving numerous C3 molecules into C3a and C3b. C3a is an anaphylatoxin, inducing inflammation. C3b, the larger fragment, plays multiple roles: it can bind to the pathogen surface, amplifying the cascade by facilitating the formation of the classical C5 convertase (C4b2a3b).
C5 Convertase and the Membrane Attack Complex (MAC):
The classical C5 convertase cleaves C5 into C5a (another anaphylatoxin) and C5b. C5b initiates the assembly of the membrane attack complex (MAC), a pore-forming structure that disrupts the pathogen's membrane, leading to cell lysis. The MAC is composed of C5b, C6, C7, C8, and multiple C9 molecules.
The Lectin Pathway: Mannose-Binding Activation
The lectin pathway is initiated by the binding of mannose-binding lectin (MBL) to carbohydrates on the surface of pathogens. MBL is a serum protein structurally similar to C1q, and this pathway shares many similarities with the classical pathway.
Initiation: MBL Binding to Pathogen Surfaces
The lectin pathway begins with the binding of MBL to mannose and other sugars found on the surface of many microorganisms. This binding is independent of antibodies, making it a crucial component of the innate immune response.
MASP Activation:
Bound MBL activates two serine proteases, MASP-1 and MASP-2, which are structurally homologous to C1r and C1s. MASP-2, like C1s, is the key enzyme responsible for cleaving C4 and C2.
C4 and C2 Cleavage and C3 Convertase Formation:
Similar to the classical pathway, MASP-2 cleaves C4 and C2, leading to the formation of the lectin C3 convertase (C4b2a). This convertase is identical in structure and function to the classical C3 convertase.
Subsequent Steps: Identical to Classical Pathway
From the formation of the C3 convertase onwards, the lectin pathway proceeds identically to the classical pathway: C3 is cleaved, C3b leads to the formation of the C5 convertase, and ultimately, the MAC is formed, causing pathogen lysis.
The Alternative Pathway: Spontaneous Activation and Amplification
The alternative pathway is unique because it can be activated spontaneously, independently of antibodies or MBL. It provides an immediate, early response to invading pathogens and is a crucial amplification loop for both the classical and lectin pathways.
Spontaneous C3 Hydrolysis:
The alternative pathway begins with the spontaneous hydrolysis of C3 in solution. This creates a small amount of C3(H2O), which is functionally similar to C3b.
Factor B and Factor D Interaction:
C3(H2O) binds to Factor B, a protein present in the serum. Factor D, a serine protease, cleaves bound Factor B into Bb and Ba. Bb remains bound to C3(H2O), forming the fluid-phase C3 convertase (C3(H2O)Bb).
Amplification Loop: C3b Deposition
The fluid-phase C3 convertase cleaves C3 into C3a and C3b. This C3b can now bind to the pathogen’s surface, initiating a positive feedback loop. Surface-bound C3b binds to Factor B, which is then cleaved by Factor D, forming the alternative C3 convertase (C3bBb).
Properdin Stabilization and C3 Convertase Activity:
Properdin (Factor P), a regulatory protein, stabilizes the alternative C3 convertase (C3bBb), extending its lifespan and amplifying C3 cleavage. This continuous C3b generation amplifies the response significantly.
C5 Convertase Formation and MAC Assembly:
The alternative C3 convertase (C3bBb) can bind another C3b molecule, forming the alternative C5 convertase (C3bBb3b). This convertase cleaves C5, leading to the formation of the MAC and subsequent pathogen lysis, mirroring the final steps of the classical and lectin pathways.
Convergence and Effector Functions: The Shared Outcomes
Despite their distinct initiation mechanisms, all three complement pathways converge at the crucial step of C3 convertase formation. This shared intermediate leads to a series of common effector functions:
-
Opsonization: C3b coats the pathogen surface, enhancing phagocytosis by cells expressing C3b receptors, such as macrophages and neutrophils.
-
Inflammation: Anaphylatoxins (C3a and C5a) stimulate mast cell degranulation, releasing histamine and other inflammatory mediators. They also attract immune cells to the site of infection.
-
Chemotaxis: C5a acts as a powerful chemoattractant, recruiting neutrophils and other phagocytes to the area of infection.
-
Cytolysis: The membrane attack complex (MAC) directly kills pathogens by creating pores in their membranes.
Regulation of the Complement System: Preventing Self-Damage
The complement system is tightly regulated to prevent inappropriate activation and damage to host cells. Several regulatory mechanisms are in place, including:
- Decay-accelerating factor (DAF): Disrupts C3 convertase activity.
- Complement receptor 1 (CR1): Inhibits C3 convertase formation and promotes C3b degradation.
- Factor I: Cleaves C3b and C4b, inhibiting further complement activation.
- Protectin (CD59): Inhibits MAC formation on host cells.
Clinical Significance: Complement Deficiencies and Diseases
Deficiencies in complement proteins can lead to increased susceptibility to infections, while uncontrolled complement activation can contribute to autoimmune diseases. Understanding complement activation is thus crucial in diagnosing and treating various conditions.
Conclusion: A Complex System with Crucial Roles
The complement system, with its three distinct activation pathways, is a vital component of the immune system. Its intricate cascade of reactions, tightly regulated to prevent self-harm, effectively eliminates pathogens and damaged cells. Understanding the intricacies of the classical, lectin, and alternative pathways is key to appreciating the body's defense mechanisms and the pathophysiology of numerous diseases. This detailed exploration aims to provide a comprehensive understanding of these pathways, equipping readers with a solid foundation in immunology. Remember, this is a complex system – mastering it requires diligent study and a thorough grasp of the individual components and their interactions.
Latest Posts
Latest Posts
-
Where Should A Food Handler Check The Temperature
Apr 01, 2025
-
Your Drivers License May Be Suspended For
Apr 01, 2025
-
Which Of The Following Is True About Major Depression
Apr 01, 2025
-
After A Child Abuse Report Is Filed
Apr 01, 2025
-
What Goods And Services Should Be Produced
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about All Three Pathways For Complement Activation Quizlet . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
