Anything That Has Mass And Takes Up Space
Breaking News Today
Mar 14, 2025 · 6 min read
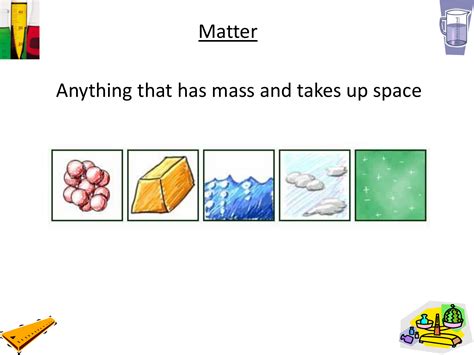
Table of Contents
Anything That Has Mass and Takes Up Space: Exploring Matter
The universe, in all its vastness and complexity, is fundamentally composed of one thing: matter. Everything you see, touch, and interact with – from the smallest speck of dust to the largest galaxy – is a manifestation of matter. But what exactly is matter? Simply put, matter is anything that has mass and occupies space. This seemingly simple definition opens the door to a fascinating exploration of the physical world, from the basic building blocks of matter to its diverse forms and behaviors.
Understanding Mass and Volume
Before delving into the intricacies of matter, let's clarify the two key properties that define it: mass and volume.
Mass: A Measure of Inertia
Mass is a measure of an object's inertia – its resistance to changes in motion. A more massive object requires more force to accelerate it than a less massive object. It's important to distinguish mass from weight. Weight is the force of gravity acting on an object's mass. An object's mass remains constant regardless of its location, while its weight can vary depending on the gravitational field it's in. For instance, an astronaut weighs less on the moon than on Earth because the moon's gravitational pull is weaker, but their mass remains unchanged.
Volume: Occupying Three-Dimensional Space
Volume is the amount of three-dimensional space that an object occupies. It's a measure of how much space an object takes up. Volume can be expressed in various units, such as cubic meters (m³), cubic centimeters (cm³), or liters (L). Understanding volume is crucial in various scientific and engineering applications, from calculating the density of materials to determining the capacity of containers.
The Building Blocks of Matter: Atoms and Molecules
Matter isn't simply a continuous substance; it's composed of incredibly tiny particles called atoms. Atoms are the fundamental building blocks of all matter, and they themselves are made up of even smaller particles: protons, neutrons, and electrons. Protons and neutrons reside in the atom's nucleus, while electrons orbit the nucleus in specific energy levels.
The number of protons in an atom's nucleus determines its atomic number and defines the element it belongs to. Elements are the pure substances that cannot be broken down into simpler substances by chemical means. Examples include hydrogen (H), oxygen (O), carbon (C), and iron (Fe). The periodic table organizes all known elements based on their atomic number and properties.
Atoms often combine to form molecules. A molecule is a group of two or more atoms chemically bonded together. For example, two hydrogen atoms combine with one oxygen atom to form a molecule of water (H₂O). The properties of molecules are often vastly different from the properties of the individual atoms that make them up. Water, for example, is a liquid at room temperature, while hydrogen and oxygen are both gases.
States of Matter: Solid, Liquid, Gas, and Plasma
Matter exists in various states, depending on the arrangement and energy of its constituent particles. The four fundamental states of matter are:
Solid
In a solid, particles are tightly packed together in a fixed arrangement, giving solids their definite shape and volume. The particles vibrate in place but don't move freely. Examples of solids include ice, rocks, and metals. Solids can be crystalline (with a regular, repeating structure) or amorphous (without a regular structure).
Liquid
In a liquid, particles are closer together than in a gas but not as tightly packed as in a solid. They can move past each other, giving liquids their ability to flow and take the shape of their container. Liquids have a definite volume but no definite shape. Examples of liquids include water, oil, and mercury.
Gas
In a gas, particles are widely dispersed and move rapidly and randomly. Gases have neither a definite shape nor a definite volume; they expand to fill their container. Examples of gases include air, helium, and carbon dioxide.
Plasma
Plasma is an electrically charged gas consisting of ions and free electrons. It's often considered the fourth state of matter and is the most common state of matter in the universe, making up stars and nebulae. Plasma is characterized by its high energy and conductivity. Examples of plasma include lightning, neon lights, and the sun.
Beyond the Four States: Bose-Einstein Condensates and Other Exotic States
While the four fundamental states of matter cover most everyday phenomena, there are other, more exotic states that exist under extreme conditions. One notable example is a Bose-Einstein condensate (BEC). A BEC is formed when a gas of bosons (a type of particle) is cooled to extremely low temperatures, causing the bosons to occupy the same quantum state and behave as a single, macroscopic entity.
Other exotic states include fermionic condensates, quantum spin liquids, and superfluids. These states exhibit fascinating properties and are subjects of ongoing research in condensed matter physics.
Properties of Matter: Physical and Chemical
Matter exhibits various properties, which can be categorized as either physical or chemical.
Physical Properties
Physical properties are characteristics that can be observed or measured without changing the substance's chemical composition. Examples include:
- Density: mass per unit volume
- Color: the wavelength of light reflected by the substance
- Melting point: the temperature at which a solid changes to a liquid
- Boiling point: the temperature at which a liquid changes to a gas
- Hardness: resistance to scratching or indentation
- Conductivity: ability to conduct heat or electricity
Chemical Properties
Chemical properties describe how a substance reacts with other substances. These properties can only be observed when a chemical change occurs, altering the substance's chemical composition. Examples include:
- Flammability: ability to burn in the presence of oxygen
- Reactivity: how readily a substance reacts with other substances
- Toxicity: the degree to which a substance is poisonous
- Acidity/Basicity: pH level of a substance
Changes in Matter: Physical and Chemical Changes
Matter can undergo two types of changes: physical and chemical.
Physical Changes
Physical changes alter the form or appearance of matter but don't change its chemical composition. Examples include:
- Changing the state of matter (e.g., melting ice)
- Dissolving sugar in water
- Crushing a rock
- Stretching a rubber band
Chemical Changes
Chemical changes, also known as chemical reactions, result in the formation of new substances with different chemical compositions. Examples include:
- Burning wood
- Rusting iron
- Digesting food
- Baking a cake
The Importance of Studying Matter
Understanding matter is crucial for numerous scientific and technological advancements. It's fundamental to:
- Material science: developing new materials with specific properties
- Chemistry: understanding chemical reactions and synthesis
- Physics: exploring the fundamental forces and laws of nature
- Biology: understanding the structure and function of living organisms
- Medicine: developing new drugs and therapies
- Engineering: designing and building structures and machines
The study of matter is a vast and constantly evolving field. As our understanding deepens, we continue to unlock new possibilities and technologies, shaping our world in profound ways. From the smallest atoms to the largest galaxies, the exploration of matter remains a central theme in our quest to comprehend the universe.
Latest Posts
Latest Posts
-
Who Can Apply Pesticides In A Food Service Establishment
Mar 14, 2025
-
Which Of The Following Is An Example Of Eustress
Mar 14, 2025
-
What Does Federal Law Say About Departmental Accountable Officials
Mar 14, 2025
-
When The Fda Conducts An Inspection The Inspectors Will
Mar 14, 2025
-
Which Of The Following Is Not Electronic Phi Ephi
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about Anything That Has Mass And Takes Up Space . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
