Cellulose And Starch Are Examples Of Quizlet
Breaking News Today
Apr 01, 2025 · 5 min read
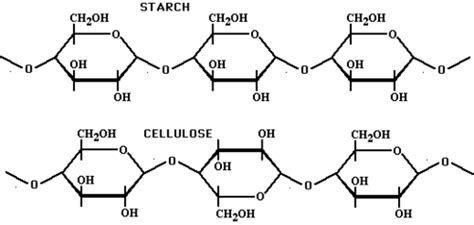
Table of Contents
Cellulose and Starch: Exploring the Differences and Similarities
Cellulose and starch are both polysaccharides, meaning they're complex carbohydrates made up of long chains of glucose units. However, despite this shared fundamental building block, their structures and functions differ significantly, leading to vastly different roles in the biological world and various industrial applications. This comprehensive exploration delves into the intricacies of cellulose and starch, comparing and contrasting their structures, properties, and uses. We'll examine their chemical compositions, explore their biological functions, and discuss their industrial significance.
Understanding Polysaccharides: The Foundation of Cellulose and Starch
Before diving into the specifics of cellulose and starch, it's crucial to grasp the broader concept of polysaccharides. These are large, complex carbohydrates formed by the joining of many monosaccharide units (simple sugars) through glycosidic bonds. These bonds link the glucose molecules in specific ways, shaping the unique properties of each polysaccharide. The type of glycosidic bond and the arrangement of glucose units are critical determinants of a polysaccharide's structure and function.
The Glucose Unit: The Common Building Block
Both cellulose and starch are composed primarily of glucose, a simple sugar essential for energy production in living organisms. The glucose molecules in both polysaccharides are linked together by glycosidic bonds, but the type of bond and the orientation of the glucose units distinguish cellulose from starch. This subtle difference in structure leads to significantly different properties.
Cellulose: The Structural Backbone of Plants
Cellulose is the most abundant organic polymer on Earth, forming the primary structural component of plant cell walls. It provides plants with their rigidity and strength, allowing them to stand tall and withstand environmental pressures.
The Structure of Cellulose: Linear Chains of Glucose
Cellulose is characterized by its linear structure, consisting of long, unbranched chains of β-D-glucose molecules. These glucose units are linked together by β(1→4) glycosidic bonds, a key structural feature that differentiates cellulose from starch. The β-linkage creates a straight chain, allowing multiple cellulose chains to align tightly parallel to each other.
Hydrogen Bonding: Strength in Numbers
The hydroxyl (-OH) groups on the glucose units in adjacent cellulose chains form strong hydrogen bonds, contributing significantly to the high tensile strength of cellulose. These hydrogen bonds create strong intermolecular forces, resulting in a highly stable and rigid structure. This robust structure makes cellulose an excellent material for providing structural support in plants.
Cellulose's Biological Significance: More Than Just Structure
Beyond its structural role in plant cell walls, cellulose also plays a crucial role in various ecological processes. It's a major component of dietary fiber in humans and other animals, promoting healthy digestion and preventing constipation. Furthermore, cellulose is a key source of carbon in the carbon cycle, making it essential for maintaining ecological balance.
Starch: The Energy Storage Powerhouse
In contrast to cellulose's structural role, starch serves primarily as an energy storage molecule in plants. It's a readily accessible energy source that plants utilize when needed for growth and metabolic processes.
The Structure of Starch: Branched and Unbranched Chains
Starch comprises two main components: amylose and amylopectin. Amylose consists of long, unbranched chains of α-D-glucose molecules linked by α(1→4) glycosidic bonds. Amylopectin, on the other hand, has a branched structure with α(1→4) linkages in the linear portions and α(1→6) branch points.
The Impact of Branching: Accessibility and Solubility
The branching in amylopectin is crucial for its functionality. The branches create more exposed ends, allowing enzymes to readily access and break down the glucose units for energy release. This branching also affects the solubility of starch, making it more easily dispersed in water compared to the less soluble amylose.
Starch's Biological Role: Energy for Plants and Animals
Starch serves as a readily available energy reserve for plants, providing energy for growth, reproduction, and various metabolic processes. It's stored in plant tissues like seeds, roots, and tubers, providing nourishment during periods of dormancy or rapid growth. Animals, including humans, also utilize starch as a crucial source of dietary energy.
Cellulose vs. Starch: A Detailed Comparison
| Feature | Cellulose | Starch |
|---|---|---|
| Monomer | β-D-glucose | α-D-glucose |
| Glycosidic Bond | β(1→4) | α(1→4) and α(1→6) (amylopectin) |
| Structure | Linear, unbranched chains | Linear (amylose) and branched (amylopectin) |
| Function | Structural support in plants | Energy storage in plants |
| Solubility | Insoluble in water | Partially soluble in water |
| Digestibility | Indigestible by humans (fiber) | Digestible by humans (energy source) |
| Hydrogen Bonding | Extensive intermolecular hydrogen bonding | Limited intermolecular hydrogen bonding |
Industrial Applications of Cellulose and Starch
Both cellulose and starch find extensive use in various industries due to their unique properties.
Cellulose: Versatile Applications
Cellulose's strength and abundance make it a valuable resource for a wide range of applications:
- Paper Production: Cellulose is the primary component of paper, providing the necessary structural integrity.
- Textiles: Cellulose is the main component of cotton, linen, and other natural fibers, used in clothing and other textiles.
- Biofuels: Cellulose can be converted into biofuels, offering a renewable energy source.
- Food Additives: Cellulose derivatives are used as thickeners, stabilizers, and emulsifiers in food products.
- Pharmaceuticals: Cellulose is used as an excipient in drug formulations.
Starch: A Multifaceted Resource
Starch's energy storage capacity and diverse chemical properties make it indispensable in many industries:
- Food Industry: Starch is used as a thickener, stabilizer, and binder in a wide array of food products, from sauces and soups to baked goods and confectionery.
- Textiles: Starch is used as a sizing agent in the textile industry to stiffen fabrics.
- Paper Production: Starch is used as a binder and adhesive in papermaking.
- Bioplastics: Starch can be used to produce biodegradable plastics, providing a sustainable alternative to traditional plastics.
- Pharmaceuticals: Starch is used as a binder and disintegrant in pharmaceutical formulations.
Conclusion: The Significance of Cellulose and Starch
Cellulose and starch, despite sharing a common glucose building block, represent distinct polysaccharides with profoundly different structures and functions. Cellulose provides crucial structural support for plants, while starch serves as a readily available energy reservoir. Both polysaccharides play vital roles in biological systems and find extensive applications across numerous industries, highlighting their importance in various aspects of human life. Further research into the properties and applications of these abundant natural polymers promises to unlock even more innovative uses in the future, contributing to the development of sustainable and environmentally friendly technologies.
Latest Posts
Latest Posts
-
The Release Of A Tendon From Adhesions
Apr 02, 2025
-
Are You Smarter Than A Fourth Grader
Apr 02, 2025
-
An 8 Month Old Infant Is Eating And Suddenly Begins To Cough
Apr 02, 2025
-
Describe How Wards And Precincts Are Part Of The Local
Apr 02, 2025
-
What Is The Full Description For Code 11001
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Cellulose And Starch Are Examples Of Quizlet . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
