Colorless Liquid Hydrocarbon Of The Alkane Series
Breaking News Today
Mar 19, 2025 · 5 min read
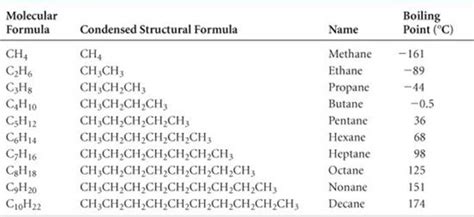
Table of Contents
Colorless Liquid Hydrocarbons of the Alkane Series: A Comprehensive Overview
Colorless liquid hydrocarbons belonging to the alkane series represent a significant portion of the petroleum and natural gas we utilize daily. Understanding their properties, applications, and environmental impact is crucial for responsible resource management and technological advancement. This comprehensive article delves into the fascinating world of these hydrocarbons, exploring their chemical characteristics, extraction methods, diverse uses, and safety considerations.
Defining Alkanes and Their Liquid Members
Alkanes, also known as paraffins, are saturated hydrocarbons, meaning they consist solely of carbon and hydrogen atoms linked together by single bonds. Their general formula is C<sub>n</sub>H<sub>2n+2</sub>, where 'n' represents the number of carbon atoms. The simplest alkanes, methane (CH<sub>4</sub>) and ethane (C<sub>2</sub>H<sub>6</sub>), are gases at room temperature. However, as the number of carbon atoms increases, the alkanes transition from gases to liquids and eventually to solids. This article focuses on the liquid alkanes, which typically range from five to sixteen carbon atoms.
Key Characteristics of Liquid Alkanes
Several key characteristics define liquid alkanes:
- Colorless and Odorless: In their pure form, they are generally colorless and odorless. Any odor detected is usually due to impurities or additives.
- Non-polar: Their non-polar nature influences their solubility and reactivity. They are insoluble in water but readily dissolve in non-polar solvents.
- Low Reactivity: Their saturated nature makes them relatively unreactive compared to unsaturated hydrocarbons like alkenes and alkynes. They primarily undergo combustion and halogenation reactions.
- Low Density: They are less dense than water, causing them to float on water's surface. This property is critical in oil spills.
- Boiling Point and Chain Length: The boiling point of liquid alkanes increases with the length of the carbon chain. Longer chains exhibit stronger intermolecular forces (London dispersion forces), leading to higher boiling points.
- Isomerism: As the number of carbon atoms increases, the possibility of isomerism (different structural arrangements of the same molecular formula) arises, leading to variations in physical and chemical properties.
Extraction and Refining of Liquid Alkanes
Liquid alkanes are primarily obtained from crude oil, a complex mixture of hydrocarbons found underground. The extraction process involves drilling wells to access oil reservoirs. Once extracted, crude oil undergoes refining to separate its various components, including liquid alkanes.
Refining Processes:
Refining utilizes several techniques, including:
- Fractional Distillation: This process separates components based on their boiling points. Crude oil is heated, and the resulting vapors are passed through a fractionating column. Components with lower boiling points condense higher in the column, while those with higher boiling points condense lower. This separates different fractions, including gasoline, kerosene, diesel, and various other liquid alkane mixtures.
- Cracking: This process breaks down larger alkane molecules into smaller, more valuable ones, such as gasoline. Thermal cracking uses high temperatures, while catalytic cracking uses catalysts to enhance efficiency.
- Isomerization: This process rearranges the structure of alkane molecules to produce isomers with improved properties, such as higher octane rating in gasoline.
Applications of Liquid Alkanes
Liquid alkanes form the backbone of numerous industries and applications. Their versatility arises from their properties, ranging from their fuel potential to their use as solvents.
Fuel Applications:
- Gasoline: A mixture of short-chain liquid alkanes and other hydrocarbons, it's the primary fuel for automobiles and other internal combustion engines.
- Kerosene: Used as jet fuel and heating oil, it contains medium-chain liquid alkanes.
- Diesel Fuel: Composed of longer-chain liquid alkanes, it powers diesel engines in vehicles and machinery.
- Propane and Butane: Although primarily gases, propane and butane can be liquefied under pressure for storage and transportation, serving as fuels for cooking, heating, and other applications.
Solvent Applications:
Liquid alkanes with longer chains are used as solvents in various industrial processes. They dissolve non-polar substances and are employed in cleaning, degreasing, and extraction procedures. Their low toxicity and relatively low environmental impact make them suitable for certain applications.
Other Applications:
- Lubricants: Long-chain alkanes are utilized in lubricants for engines and machinery, reducing friction and wear.
- Plastics and Polymers: Some alkanes are used as feedstock for the production of plastics and polymers.
- Waxes: Long-chain alkanes form the basis of paraffin waxes used in candles, coatings, and other applications.
- Cosmetics and Pharmaceuticals: Certain alkanes find application in the cosmetic and pharmaceutical industries, often as components of creams, lotions, and ointments.
Environmental Considerations and Safety
While liquid alkanes are essential to modern life, their extraction, processing, and use pose environmental and safety challenges.
Environmental Impacts:
- Greenhouse Gas Emissions: The combustion of liquid alkanes releases carbon dioxide (CO2), a significant greenhouse gas contributing to climate change.
- Oil Spills: Accidental spills during extraction, transportation, or storage can cause significant environmental damage, impacting marine life and coastal ecosystems.
- Air Pollution: Incomplete combustion of liquid alkanes can release harmful pollutants like particulate matter, carbon monoxide, and nitrogen oxides, contributing to air quality issues.
Safety Precautions:
- Flammability: Liquid alkanes are highly flammable, requiring careful handling and storage to prevent fires and explosions. Proper ventilation and adherence to safety regulations are crucial.
- Toxicity: While generally considered less toxic than other chemicals, prolonged exposure to certain liquid alkanes can have adverse health effects. Proper protective equipment and ventilation should be used.
- Environmental Regulations: Strict environmental regulations govern the extraction, refining, and disposal of liquid alkanes, aiming to minimize their environmental footprint.
Future Trends and Research
Ongoing research and development focus on several aspects of liquid alkanes:
- Sustainable Extraction Methods: Exploration of techniques to minimize environmental damage associated with oil extraction is a high priority.
- Alternative Fuels: Research into alternative fuels, including biofuels and hydrogen, aims to reduce dependence on fossil fuels and mitigate climate change.
- Improved Refining Processes: Efforts continue to develop more efficient and environmentally friendly refining processes, minimizing waste and emissions.
- Enhanced Combustion Technologies: Technologies designed to improve combustion efficiency and reduce pollutant emissions are continuously being developed.
Conclusion
Colorless liquid hydrocarbons of the alkane series are indispensable components of our modern world, providing fuel, solvents, and numerous other essential materials. Understanding their chemical properties, extraction methods, and diverse applications is key to ensuring their responsible use. Simultaneously, ongoing research and development efforts are vital for minimizing environmental impacts and exploring sustainable alternatives to meet future energy demands and reduce the risks associated with these invaluable resources. By addressing environmental concerns and prioritizing safety, we can continue to leverage the benefits of liquid alkanes while minimizing their negative consequences.
Latest Posts
Latest Posts
-
Permanent Colors Containing Para Dyes Would Fall Into Which Color Category
Mar 19, 2025
-
Identify One Social Factor That Influenced American Imperialism
Mar 19, 2025
-
The Court Discovered Right To Implict In The Shawdows
Mar 19, 2025
-
When Does The Engage Stage Of The Inbound Methodology Begin
Mar 19, 2025
-
Locally Installed Software Is Updated Automatically By The Software Developer
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Colorless Liquid Hydrocarbon Of The Alkane Series . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
