Compare And Contrast The Single And Double Replacement Reactions.
Breaking News Today
Mar 14, 2025 · 6 min read
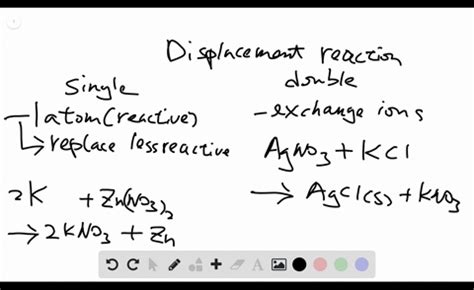
Table of Contents
Single vs. Double Replacement Reactions: A Comprehensive Comparison
Chemical reactions are the fundamental processes that govern the transformation of matter. Among the diverse types of reactions, single and double replacement reactions stand out as common and crucial processes in various fields, from industrial chemistry to biological systems. While seemingly similar at first glance, these two reaction types exhibit distinct characteristics and mechanisms. This comprehensive article delves into a detailed comparison and contrast of single and double replacement reactions, exploring their definitions, mechanisms, examples, and applications.
Understanding Single Replacement Reactions
A single replacement reaction, also known as a single displacement reaction, is a type of chemical reaction where one element replaces another element in a compound. This reaction typically involves a more reactive element displacing a less reactive element from its compound. The general form of a single replacement reaction can be represented as:
A + BC → AC + B
where:
- A represents a more reactive element.
- BC represents a compound.
- AC represents a new compound formed.
- B represents the less reactive element displaced.
The reactivity of elements is typically determined by their position in the activity series (also known as the reactivity series), a list of elements ordered by their tendency to undergo oxidation (loss of electrons). An element higher in the activity series will readily displace an element lower in the series.
Mechanism of Single Replacement Reactions
Single replacement reactions often involve the transfer of electrons between the reactants. The more reactive element (A) loses electrons (oxidation) and becomes a cation, while the less reactive element (B) gains electrons (reduction) and becomes an anion or a neutral atom. This electron transfer is the driving force behind the reaction. The reaction proceeds spontaneously if the change in Gibbs free energy (ΔG) is negative, indicating a thermodynamically favorable process.
Examples of Single Replacement Reactions
Several classic examples illustrate the concept of single replacement reactions:
-
Reaction of zinc with hydrochloric acid: Zinc (Zn), being more reactive than hydrogen (H), displaces hydrogen from hydrochloric acid (HCl), producing zinc chloride (ZnCl₂) and hydrogen gas (H₂):
Zn(s) + 2HCl(aq) → ZnCl₂(aq) + H₂(g)
-
Reaction of iron with copper(II) sulfate: Iron (Fe) is more reactive than copper (Cu), leading to the displacement of copper from copper(II) sulfate (CuSO₄), resulting in the formation of iron(II) sulfate (FeSO₄) and copper metal (Cu):
Fe(s) + CuSO₄(aq) → FeSO₄(aq) + Cu(s)
-
Reaction of chlorine with sodium bromide: Chlorine (Cl₂), a more reactive halogen than bromine (Br₂), displaces bromine from sodium bromide (NaBr), forming sodium chloride (NaCl) and bromine:
Cl₂(g) + 2NaBr(aq) → 2NaCl(aq) + Br₂(l)
These examples highlight the essential characteristic of single replacement reactions: one element replacing another within a compound, dictated by their relative reactivities.
Understanding Double Replacement Reactions
A double replacement reaction, also known as a double displacement reaction or metathesis reaction, involves the exchange of ions between two compounds. This reaction typically occurs in aqueous solutions, where the reactants are dissolved in water, leading to the formation of two new compounds. The general form of a double replacement reaction is:
AB + CD → AD + CB
where:
- AB and CD represent two ionic compounds.
- AD and CB represent the two new ionic compounds formed.
Mechanism of Double Replacement Reactions
In double replacement reactions, the cations and anions of the reactant compounds switch partners. The driving force for these reactions is often the formation of a precipitate (an insoluble solid), a gas, or a weak electrolyte (a compound that does not readily dissociate into ions in solution). If none of these are formed, the reaction may not proceed significantly.
Examples of Double Replacement Reactions
Several examples illustrate the various outcomes of double replacement reactions:
-
Precipitation reaction: The reaction between silver nitrate (AgNO₃) and sodium chloride (NaCl) produces a precipitate of silver chloride (AgCl), an insoluble compound, and soluble sodium nitrate (NaNO₃):
AgNO₃(aq) + NaCl(aq) → AgCl(s) + NaNO₃(aq)
-
Gas-forming reaction: The reaction between hydrochloric acid (HCl) and sodium carbonate (Na₂CO₃) produces carbon dioxide gas (CO₂), water (H₂O), and sodium chloride (NaCl):
2HCl(aq) + Na₂CO₃(aq) → 2NaCl(aq) + H₂O(l) + CO₂(g)
-
Neutralization reaction (a specific type of double replacement): The reaction between an acid and a base, such as hydrochloric acid (HCl) and sodium hydroxide (NaOH), produces water (H₂O) and a salt (NaCl):
HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l)
Conditions for Double Replacement Reactions to Occur
Several conditions must be met for a double replacement reaction to occur:
-
Formation of a precipitate: The most common driving force is the formation of an insoluble solid that precipitates out of the solution. Solubility rules are used to predict whether a precipitate will form.
-
Formation of a gas: The production of a gas, such as carbon dioxide or hydrogen sulfide, drives the reaction forward.
-
Formation of water: The formation of water, as seen in neutralization reactions, is another driving force.
If none of these conditions are met, the reaction may not proceed to a significant extent. The reactants remain largely as they are, with minimal ion exchange.
Comparing and Contrasting Single and Double Replacement Reactions
Both single and double replacement reactions involve the rearrangement of atoms and ions, but their mechanisms and driving forces differ significantly:
| Feature | Single Replacement Reaction | Double Replacement Reaction |
|---|---|---|
| Type of Reaction | One element replaces another in a compound | Ions exchange partners between two compounds |
| General Form | A + BC → AC + B | AB + CD → AD + CB |
| Driving Force | Relative reactivity of elements; electron transfer | Formation of precipitate, gas, or weak electrolyte |
| Number of Reactants | Two | Two |
| Number of Products | Two | Two |
| Typical State of Reactants | Can be solid, liquid, or gas; often involves an aqueous solution | Typically aqueous solutions |
| Example | Zn(s) + 2HCl(aq) → ZnCl₂(aq) + H₂(g) | AgNO₃(aq) + NaCl(aq) → AgCl(s) + NaNO₃(aq) |
Applications of Single and Double Replacement Reactions
Both single and double replacement reactions find widespread applications across various fields:
Applications of Single Replacement Reactions
-
Metal extraction: Single replacement reactions are crucial in extracting metals from their ores. More reactive metals are used to displace less reactive metals from their compounds.
-
Corrosion: The rusting of iron is a classic example of a single replacement reaction where oxygen in the air displaces iron from its protective oxide layer.
-
Electroplating: This process involves using a single replacement reaction to coat a metal object with a thin layer of another metal, improving its appearance or corrosion resistance.
Applications of Double Replacement Reactions
-
Water softening: Double replacement reactions are used to remove unwanted ions from water, such as calcium and magnesium ions, making it softer.
-
Wastewater treatment: These reactions play a crucial role in removing heavy metals and other pollutants from wastewater.
-
Chemical synthesis: Double replacement reactions are employed in the synthesis of a vast range of chemical compounds. Many important drugs and other chemicals are produced using these reactions.
Conclusion
Single and double replacement reactions, although both involving the rearrangement of atoms, differ significantly in their mechanisms and driving forces. Single replacement reactions are driven by the relative reactivity of elements and involve the transfer of electrons, while double replacement reactions are driven by the formation of a precipitate, gas, or weak electrolyte and involve the exchange of ions. Both reaction types play crucial roles in various chemical processes and have widespread applications in different industries and fields of study. Understanding these reactions is essential for comprehending fundamental chemical principles and their practical applications in the world around us.
Latest Posts
Latest Posts
-
Which Of The Following Scenarios Describe A Potential Insider Threat
Mar 14, 2025
-
After A Classified Document Is Leaked Online
Mar 14, 2025
-
Which Of The Following Represents Critical Information
Mar 14, 2025
-
Fundamentals Of Physics 12 Edition Instructors Solutions Manual Pdf
Mar 14, 2025
-
What Is Photosynthesis Check All That Apply
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about Compare And Contrast The Single And Double Replacement Reactions. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
