Complete The Overall Reaction Catalyzed By The Pyruvate Dehydrogenase Complex
Breaking News Today
Mar 30, 2025 · 5 min read
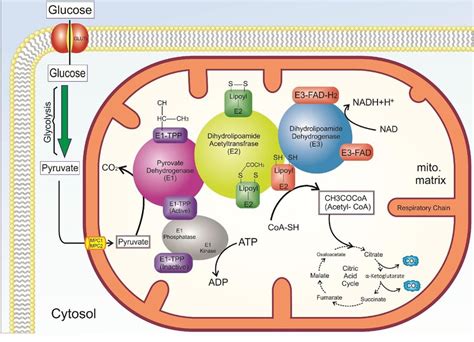
Table of Contents
The Pyruvate Dehydrogenase Complex: A Complete Overview of the Reaction
The pyruvate dehydrogenase complex (PDC) is a magnificent molecular machine, a crucial enzyme complex residing within the mitochondrial matrix of eukaryotic cells and the cytoplasm of prokaryotes. Its primary function is to catalyze the irreversible oxidative decarboxylation of pyruvate, the end product of glycolysis, into acetyl-CoA. This reaction is a pivotal link between glycolysis and the citric acid cycle (Krebs cycle), a central pathway in cellular respiration responsible for generating the majority of ATP, the cell's energy currency. Understanding the complete reaction catalyzed by the PDC is essential to grasping the intricate workings of cellular metabolism.
The Overall Reaction: A Summary
Before diving into the intricacies of each step, let's establish the overall reaction catalyzed by the pyruvate dehydrogenase complex:
Pyruvate + CoA-SH + NAD⁺ → Acetyl-CoA + CO₂ + NADH + H⁺
This seemingly simple equation masks a complex series of reactions involving multiple enzymes and coenzymes working in a highly coordinated manner. The reaction involves the removal of a carboxyl group from pyruvate as carbon dioxide (CO₂), the oxidation of the remaining two-carbon fragment, and the subsequent formation of acetyl-CoA, a crucial molecule that feeds into the citric acid cycle. The reduction of NAD⁺ to NADH generates reducing equivalents, crucial for ATP production through oxidative phosphorylation.
The Three Enzymes and Their Cofactors: A Detailed Look
The pyruvate dehydrogenase complex is a massive assembly composed of three distinct enzymes: pyruvate dehydrogenase (E1), dihydrolipoyl transacetylase (E2), and dihydrolipoyl dehydrogenase (E3). Each enzyme plays a crucial role, and their coordinated activity is essential for the successful completion of the overall reaction. Furthermore, several crucial cofactors are involved in the catalytic process.
1. Pyruvate Dehydrogenase (E1): The Initiator
E1, a thiamine pyrophosphate (TPP)-dependent enzyme, catalyzes the first step of the reaction. TPP, a derivative of vitamin B1, acts as a crucial cofactor in this step. Here's a breakdown:
- Decarboxylation: E1 binds pyruvate and catalyzes its decarboxylation, releasing CO₂. This step is facilitated by the carbanion formed on TPP, which acts as an electron sink, stabilizing the negatively charged intermediate.
- Hydroxyethyl-TPP Formation: The remaining two-carbon fragment, a hydroxyethyl group, remains bound to TPP.
2. Dihydrolipoyl Transacetylase (E2): The Connector
E2 is the central enzyme within the complex and utilizes lipoic acid, a covalently attached cofactor, to facilitate the next steps. Here's its role:
- Oxidative Decarboxylation: The hydroxyethyl group bound to TPP is transferred to the oxidized lipoyl group of E2. This transfer involves an oxidation of the hydroxyethyl group to an acetyl group, reducing the lipoyl group to its dihydrolipoyl form.
- Acetyl-CoA Formation: The acetyl group is then transferred from the dihydrolipoyl group to Coenzyme A (CoA-SH), forming acetyl-CoA, the final product of this stage. This step utilizes the high-energy thioester bond of CoA.
3. Dihydrolipoyl Dehydrogenase (E3): The Regenerator
E3, a flavoprotein containing flavin adenine dinucleotide (FAD) as a prosthetic group, is responsible for regenerating the oxidized lipoyl group of E2.
- Reoxidation of Lipoic Acid: E3 catalyzes the reoxidation of the dihydrolipoyl group of E2 using FAD as an electron acceptor. This step reduces FAD to FADH2.
- NADH Production: Finally, FADH2 reduces NAD⁺ to NADH, regenerating the oxidized lipoyl group and completing the catalytic cycle.
Regulation of the Pyruvate Dehydrogenase Complex: A Crucial Control Point
The activity of the pyruvate dehydrogenase complex is tightly regulated to ensure that the flow of metabolites through the citric acid cycle is coordinated with the cell's energy needs. Several factors influence the regulation:
- Product Inhibition: High levels of acetyl-CoA and NADH inhibit the complex, slowing down the production of acetyl-CoA when the citric acid cycle is already operating at a high capacity.
- Substrate Availability: The availability of pyruvate and CoA-SH influences the activity of the complex. Low levels of these substrates lead to reduced activity.
- Phosphorylation/Dephosphorylation: A critical regulatory mechanism involves the phosphorylation and dephosphorylation of E1. Phosphorylation by pyruvate dehydrogenase kinase inactivates the complex, whereas dephosphorylation by pyruvate dehydrogenase phosphatase activates it. The balance between kinase and phosphatase activity is influenced by several factors, including the energy charge of the cell. High energy charge (high ATP/ADP ratio) favors phosphorylation and inactivation, whereas low energy charge favors dephosphorylation and activation.
Clinical Significance: Diseases Associated with PDC Deficiency
Deficiencies in the pyruvate dehydrogenase complex or its associated cofactors can lead to severe metabolic disorders, commonly referred to as pyruvate dehydrogenase complex deficiencies. These genetic disorders result in a buildup of pyruvate and lactate in the body, leading to various neurological and developmental problems. Symptoms can vary widely depending on the specific enzyme or cofactor affected and the severity of the deficiency. These disorders often manifest in early childhood and can lead to significant developmental delays, seizures, and even death.
Conclusion: The PDC, a Metabolic Masterpiece
The pyruvate dehydrogenase complex stands as a testament to the sophistication and elegance of cellular machinery. Its intricate multi-enzyme structure, the precise choreography of its enzymatic steps, and its sophisticated regulatory mechanisms underscore its pivotal role in cellular respiration. By meticulously coordinating the conversion of pyruvate to acetyl-CoA, the PDC provides a seamless transition from glycolysis to the citric acid cycle, ensuring the efficient generation of ATP, the driving force behind virtually all cellular processes. Furthermore, a thorough understanding of this complex is essential for comprehending the metabolic basis of various diseases and for developing potential therapeutic interventions. Further research continues to unravel the intricate details of PDC function and regulation, revealing even more about this remarkable molecular machine. The exploration of its intricate workings remains a key area of research, holding the promise of breakthroughs in metabolic disease treatment and understanding the fundamental principles of cellular energy production. The continued study of the PDC and its regulation offers exciting opportunities to advance our understanding of human metabolism and develop new strategies for combating metabolic disorders.
Latest Posts
Latest Posts
-
Job Specifications Are Often Referred To As
Apr 01, 2025
-
Which Of The Following Is An Example Of Indexing
Apr 01, 2025
-
King Uses The Check And Promissory Note Metaphors To
Apr 01, 2025
-
Poor Maintenance Of Home Poor Personal Care
Apr 01, 2025
-
Which Statement Is True About The Factors Affecting Physical Fitness
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Complete The Overall Reaction Catalyzed By The Pyruvate Dehydrogenase Complex . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
