Continuing Review Of An Approved And Ongoing Study
Breaking News Today
Mar 29, 2025 · 6 min read
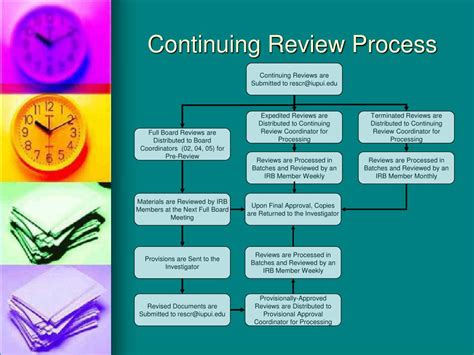
Table of Contents
Continuing Review of an Approved and Ongoing Study: A Comprehensive Guide
Conducting research ethically and responsibly necessitates a rigorous and ongoing commitment to maintaining the highest standards. This is particularly true for studies that extend over an extended period. A crucial element of this commitment is the continuing review process for approved and ongoing studies. This process ensures the continued safety, wellbeing, and rights of participants while also guaranteeing the study's scientific integrity. This comprehensive guide delves into the intricacies of continuing review, addressing key aspects, best practices, and potential challenges.
Understanding Continuing Review: The Foundation of Ethical Research
Continuing review, often referred to as continuing review of an approved and ongoing study, is a systematic and periodic evaluation of research projects that have already received initial approval from an Institutional Review Board (IRB) or ethics committee. It's not a mere formality; it's a vital safeguard against potential risks and a commitment to upholding ethical principles throughout the study's duration. The frequency of review varies depending on the nature of the research, the potential risks involved, and the IRB's policies. However, it's generally conducted annually or at other predetermined intervals.
Key Objectives of Continuing Review:
- Participant Safety and Wellbeing: The primary focus is on ensuring that participants remain protected from harm throughout the research process. This involves assessing the ongoing risks, benefits, and any unforeseen adverse events.
- Data Integrity and Scientific Rigor: Continuing review ensures that the study maintains its scientific integrity, adhering to its original design and methodology. It prevents deviations that could compromise the validity and reliability of the results.
- Compliance with Regulations and Ethical Guidelines: The review process verifies adherence to all relevant regulations, ethical guidelines, and IRB policies. This includes compliance with informed consent procedures, data privacy regulations, and reporting requirements.
- Identifying and Addressing Problems: It provides a mechanism for detecting and resolving any problems or issues that may arise during the study's implementation. This proactive approach helps prevent serious ethical breaches or compromising the integrity of the research.
The Continuing Review Process: A Step-by-Step Guide
The continuing review process typically involves several key steps, though specific procedures may vary slightly between institutions.
1. The Continuing Review Application:
This is the formal submission made by the researchers to the IRB. It includes:
- Progress Report: A detailed account of the study's progress, including enrollment numbers, data collection methods, any significant findings, and challenges encountered.
- Adverse Event Report: A comprehensive documentation of any adverse events (unexpected problems or injuries) experienced by participants. This is crucial for identifying potential risks and making necessary changes.
- Changes to the Protocol: If any changes have been made to the research protocol since the initial approval (e.g., modifications to the methodology, inclusion/exclusion criteria, or informed consent process), these must be fully documented and justified.
- Consent Forms and Materials: Review of current consent forms to ensure they're still appropriate and adequately inform participants about the study's procedures, risks, and benefits.
- Recruitment and Retention: An explanation of recruitment and retention rates, addressing any difficulties in recruiting or keeping participants. This could lead to significant implications for the study's validity.
2. IRB Review and Evaluation:
The IRB carefully examines the continuing review application. They assess the following:
- Participant Safety: The IRB evaluates the ongoing risks to participants, ensuring that the benefits outweigh the potential harms. Any changes to risks need careful evaluation.
- Data Integrity: They examine the data collected to ensure its accuracy and reliability. Any potential biases or inconsistencies must be addressed.
- Compliance: The IRB verifies that the study remains in compliance with all applicable regulations, guidelines, and policies.
- Protocol Adherence: They assess whether the study is adhering to its original protocol and if any deviations are justified.
3. IRB Decision:
After their thorough review, the IRB makes a decision regarding the continued approval of the study. The possible decisions include:
- Full Approval: The study can continue as planned.
- Approval with Modifications: The study can continue but with specific changes or requirements to address identified issues.
- Suspension: The study is temporarily halted until the researchers address the identified concerns.
- Termination: The study is terminated due to serious ethical violations or irreconcilable problems.
4. Post-Review Actions:
Depending on the IRB's decision, the researchers take necessary steps. This might involve:
- Implementing modifications: Making changes to the study protocol, informed consent documents, or data collection procedures.
- Addressing adverse events: Developing strategies to mitigate or prevent future adverse events.
- Providing updates to participants: Informing participants of any relevant changes to the study.
- Submitting progress reports and additional documentation as required.
Challenges in Continuing Review and Best Practices
While crucial, the continuing review process can present various challenges.
Challenges:
- Administrative Burden: Preparing and submitting the necessary documentation can be time-consuming and resource-intensive.
- Evolving Research Landscape: The research context may change over time, requiring adjustments to the study protocol and posing challenges in maintaining ethical oversight.
- Maintaining Participant Engagement: Longitudinal studies may experience challenges in maintaining participant engagement and adherence.
- Unforeseen Circumstances: Unexpected events or changes can necessitate modifications to the study protocol and require careful evaluation by the IRB.
Best Practices:
- Proactive Monitoring: Researchers should proactively monitor participant safety and data integrity throughout the study, identifying and addressing any potential problems early.
- Thorough Documentation: Meticulous record-keeping is essential to ensure transparent and accountable research.
- Open Communication: Maintaining open communication with the IRB and participants is crucial for addressing concerns promptly.
- Regular Training: Researchers and study staff should receive regular training on ethical research conduct and IRB requirements.
- Utilizing Technology: Employing technology for data management, participant communication, and adverse event reporting can streamline the process and enhance efficiency.
Specific Considerations for Different Research Designs
The continuing review process needs to adapt to the specifics of different research methodologies.
Longitudinal Studies:
These studies span several years, requiring more frequent reviews and close attention to participant attrition and maintaining confidentiality over extended periods.
Intervention Studies:
These studies need careful monitoring of treatment effects and side effects, with a focus on participant safety and reporting of adverse events.
Qualitative Research:
While less focused on numerical data, qualitative studies require careful review of data collection methods, participant confidentiality, and potential risks associated with sensitive topics.
Conclusion: A Continuous Commitment to Ethical Research
The continuing review of an approved and ongoing study is not just a procedural requirement; it’s an unwavering commitment to ethical research practices. It serves as a vital mechanism to safeguard participant welfare, maintain data integrity, and ensure adherence to regulations and ethical guidelines. By embracing best practices and proactively addressing challenges, researchers can effectively navigate the continuing review process and maintain the highest standards of ethical conduct throughout the lifecycle of their research projects. The ultimate goal is to foster trust, protect participants, and generate reliable and meaningful scientific knowledge.
Latest Posts
Latest Posts
-
The Great Compromise Did All Of The Following Except
Mar 31, 2025
-
Acq 0030 Overview Of Acquisition Ethics Test Answers Quizlet
Mar 31, 2025
-
A Normal Level Of Consciousness In An Infant Quizlet
Mar 31, 2025
-
A Republic Is A Form Of Government Where Quizlet
Mar 31, 2025
-
According To The Diathesis Stress Model Of Schizophrenia Quizlet
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Continuing Review Of An Approved And Ongoing Study . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
