Conversion Factors And Problem Solving Lab 2 Report Sheet Answers
Breaking News Today
Mar 14, 2025 · 5 min read
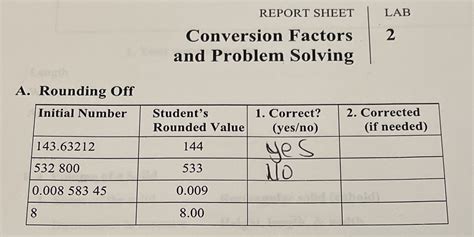
Table of Contents
Conversion Factors and Problem Solving Lab 2: A Comprehensive Guide
This report delves into the crucial concept of conversion factors and their application in problem-solving, specifically addressing common challenges encountered in a typical Chemistry Lab 2 setting. We'll explore various examples, providing detailed explanations and solutions to help you understand and master this essential skill. This guide will act as your comprehensive resource for tackling conversion factor problems, enhancing your understanding of dimensional analysis, and ultimately, improving your lab report scores.
Understanding Conversion Factors
Conversion factors are the bridge between different units of measurement. They are ratios equal to one, allowing us to convert a quantity from one unit to another without changing its value. The key is understanding the relationship between the units involved. For example, we know that 1 meter equals 100 centimeters. Therefore, we can create two conversion factors:
- 1 meter/100 centimeters (or 1 m/100 cm)
- 100 centimeters/1 meter (or 100 cm/1 m)
The choice of which factor to use depends on the desired outcome. If we want to convert centimeters to meters, we use the first factor. If we want to convert meters to centimeters, we use the second. This is the core principle of dimensional analysis, a powerful problem-solving technique.
Mastering Dimensional Analysis
Dimensional analysis, also known as the factor-label method, is a systematic approach to solving conversion problems. It involves strategically multiplying the given quantity by conversion factors to cancel out unwanted units and arrive at the desired units. The process ensures that your calculations are not only accurate but also easily understandable. Here's a step-by-step breakdown:
-
Identify the given quantity and its units. This is your starting point.
-
Identify the desired units. This is your target.
-
Find appropriate conversion factors. You may need multiple factors to bridge the gap between the given and desired units.
-
Set up the problem. Arrange the conversion factors so that unwanted units cancel out, leaving only the desired units.
-
Calculate the answer. Perform the necessary multiplications and divisions.
-
Check your answer. Ensure the units are correct and the numerical value is reasonable.
Common Conversion Factor Problems and Solutions
Let's tackle some common problems encountered in a Chemistry Lab 2 setting:
Problem 1: Converting Mass Units
Problem: Convert 250 grams of sodium chloride (NaCl) to kilograms.
Solution:
- Given: 250 g NaCl
- Desired: kg NaCl
- Conversion Factor: 1 kg = 1000 g
250 g NaCl × (1 kg / 1000 g) = 0.25 kg NaCl
Problem 2: Converting Volume Units
Problem: Convert 500 milliliters (mL) of water to liters (L).
Solution:
- Given: 500 mL water
- Desired: L water
- Conversion Factor: 1 L = 1000 mL
500 mL water × (1 L / 1000 mL) = 0.5 L water
Problem 3: Converting Units of Concentration (Molarity)
Problem: A solution has a concentration of 2.5 moles of solute per liter of solution (2.5 M). How many moles of solute are present in 250 mL of this solution?
Solution:
- Given: 2.5 mol/L, 250 mL
- Desired: mol solute
- Conversion Factors: 1 L = 1000 mL
250 mL × (1 L / 1000 mL) × (2.5 mol / 1 L) = 0.625 mol solute
Problem 4: Multi-Step Conversions
Problem: Convert 150 centimeters to kilometers.
Solution: This requires two conversion factors.
- Given: 150 cm
- Desired: km
- Conversion Factors: 100 cm = 1 m, 1000 m = 1 km
150 cm × (1 m / 100 cm) × (1 km / 1000 m) = 0.0015 km
Problem 5: Conversions Involving Density
Problem: The density of ethanol is 0.789 g/mL. What is the mass of 250 mL of ethanol?
Solution:
- Given: 0.789 g/mL, 250 mL
- Desired: g ethanol
- Conversion Factor: Density itself acts as the conversion factor.
250 mL × (0.789 g / 1 mL) = 197.25 g ethanol
Advanced Applications and Considerations
Beyond basic unit conversions, conversion factors are indispensable in various chemical calculations:
-
Stoichiometry: Conversion factors derived from balanced chemical equations are used to calculate the amounts of reactants and products involved in chemical reactions.
-
Gas Laws: Conversion factors are crucial for working with gas volumes, pressures, and temperatures in different units (e.g., converting Celsius to Kelvin).
-
Thermochemistry: Converting energy units (e.g., joules to calories) is often necessary in thermochemical calculations.
-
Solution Chemistry: Conversion factors are essential for preparing solutions of specific concentrations, involving molarity, molality, and other concentration units.
Dealing with Significant Figures
Remember to pay close attention to significant figures throughout your calculations. The final answer should reflect the precision of the given data. Your lab report will be marked down if you don’t accurately reflect significant figures.
Error Analysis
A crucial aspect of any lab report is a thorough error analysis. Discuss possible sources of error in your experiment and how these errors might have affected your results. This demonstrates a deeper understanding of the experimental process.
Conclusion: Mastering Conversion Factors for Lab Success
Conversion factors are fundamental tools in chemistry and are essential for success in your Chemistry Lab 2. Mastering dimensional analysis and applying the techniques discussed in this report will significantly improve your ability to solve a wide range of problems accurately and efficiently. By meticulously following the steps, paying attention to significant figures, and conducting a thorough error analysis, you'll not only achieve higher scores on your lab reports but also gain a deeper understanding of the underlying principles of chemical calculations. Practice consistently, and you'll become proficient in using conversion factors to unravel complex chemical problems. Remember, the key is systematic application and a clear understanding of the relationships between units.
Latest Posts
Latest Posts
-
Mandy Will Need To Extend Her Ladder To 60 Feet
May 09, 2025
-
Southern And Eastern Asia Physical Features Map
May 09, 2025
-
Which Is A Result Of Island Hopping
May 09, 2025
-
The Core Element Of Every Play Is
May 09, 2025
-
During The Meuse Argonne Offensive Of 1918 The Americans Helped
May 09, 2025
Related Post
Thank you for visiting our website which covers about Conversion Factors And Problem Solving Lab 2 Report Sheet Answers . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.