Draw The Major Product Of This Reaction. Ignore Inorganic Byproducts.
Breaking News Today
Mar 24, 2025 · 5 min read
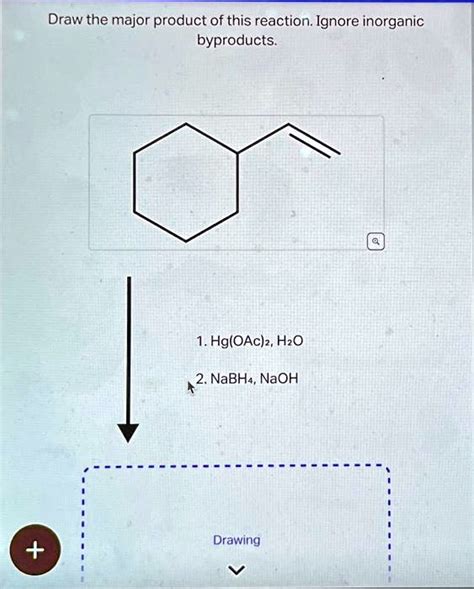
Table of Contents
Draw the Major Product of This Reaction: A Comprehensive Guide to Organic Reaction Mechanisms
Predicting the major product of an organic reaction requires a deep understanding of reaction mechanisms, reaction kinetics, and the principles of organic chemistry. This article delves into the process of determining the major product, focusing on various reaction types and the factors influencing product selectivity. We'll explore different reaction mechanisms, providing examples and explanations to build your predictive capabilities. We'll ignore inorganic byproducts throughout.
Understanding Reaction Mechanisms: The Key to Predicting Products
Before predicting the major product, it's crucial to understand the underlying reaction mechanism. The mechanism details the step-by-step process of bond breaking and bond formation, revealing the pathway leading to product formation. Key concepts to master include:
1. Nucleophilic Substitution Reactions (SN1 and SN2)
-
SN2 Reactions: These are concerted reactions, meaning bond breaking and bond formation occur simultaneously. The nucleophile attacks the substrate from the backside, leading to inversion of configuration at the stereocenter. Steric hindrance significantly impacts the reaction rate; bulky substrates react slower. Strong nucleophiles favor SN2 reactions.
-
SN1 Reactions: These are two-step reactions. The first step involves the formation of a carbocation intermediate, followed by nucleophilic attack. The carbocation intermediate is planar, leading to racemization (a mixture of stereoisomers) at the stereocenter. Weak nucleophiles and stable carbocations favor SN1 reactions.
Example: Consider the reaction of 2-bromobutane with sodium hydroxide (NaOH) in ethanol. NaOH is a strong nucleophile, favoring SN2. The major product will be 2-butanol with inverted stereochemistry.
Draw the major product: The structure will depend on the starting stereochemistry of 2-bromobutane. If the starting material is (R)-2-bromobutane, the product will be (S)-2-butanol.
2. Electrophilic Addition Reactions
These reactions are common for alkenes and alkynes. An electrophile attacks the double or triple bond, forming a carbocation intermediate (in many cases). A nucleophile then attacks the carbocation, leading to the final product. Markovnikov's rule predicts the regioselectivity (where the electrophile adds) – the electrophile adds to the carbon atom with more hydrogen atoms.
Example: The addition of HBr to propene. The HBr adds across the double bond; the hydrogen atom adds to the carbon with more hydrogens (Markovnikov's rule). The major product is 2-bromopropane.
Draw the major product: Draw the propene structure. Add a hydrogen atom to one carbon of the double bond and a bromine atom to the other, following Markovnikov's rule.
3. Elimination Reactions (E1 and E2)
-
E2 Reactions: These are concerted reactions where the base abstracts a proton and the leaving group departs simultaneously, forming a double bond. The stereochemistry plays a significant role; anti-periplanar arrangement of the proton and the leaving group is favored.
-
E1 Reactions: These are two-step reactions involving the formation of a carbocation intermediate followed by base-induced proton abstraction. The carbocation can lead to the formation of multiple alkenes (regioselectivity and stereoselectivity). Zaitsev's rule predicts the major product – the alkene with the most substituted double bond will be favored.
Example: The reaction of 2-bromo-2-methylbutane with potassium tert-butoxide (t-BuOK). t-BuOK is a strong, bulky base, favoring E2 elimination. The major product, according to Zaitsev's rule, will be 2-methyl-2-butene (the most substituted alkene).
Draw the major product: Draw 2-bromo-2-methylbutane. Remove a hydrogen and a bromine, forming a double bond to create the most substituted alkene, 2-methyl-2-butene.
4. Addition Reactions to Carbonyl Compounds
Nucleophiles attack the electrophilic carbonyl carbon, forming a tetrahedral intermediate. The reaction pathway then depends on the nature of the nucleophile and the reaction conditions.
Example: The reaction of an aldehyde with a Grignard reagent. The Grignard reagent acts as a nucleophile, attacking the carbonyl carbon. After an acidic workup, the major product will be a secondary alcohol.
Draw the major product: Draw the aldehyde and the Grignard reagent. Show the nucleophilic attack of the Grignard reagent on the carbonyl carbon. After protonation, you'll obtain a secondary alcohol.
5. Oxidation and Reduction Reactions
These reactions involve changes in the oxidation state of carbon atoms. Oxidizing agents increase the oxidation state (e.g., converting alcohols to ketones or carboxylic acids), while reducing agents decrease the oxidation state (e.g., converting ketones to alcohols).
Example: The oxidation of a secondary alcohol with chromic acid (H2CrO4). The major product will be a ketone.
Draw the major product: Draw the secondary alcohol. Oxidize the alcohol by replacing the -OH group with a =O group to form a ketone.
Factors Influencing Product Selectivity
Several factors can influence the major product formed in a reaction:
-
Steric Hindrance: Bulky groups can hinder nucleophilic attack or base abstraction, influencing reaction rates and product selectivity.
-
Electronic Effects: Electron-donating or electron-withdrawing groups can affect the reactivity of the substrate and the stability of intermediates.
-
Reaction Conditions: Temperature, solvent, and concentration can significantly impact reaction pathways and product distribution.
-
Catalyst: Catalysts can influence the reaction pathway, leading to different products.
Advanced Concepts and Considerations
-
Thermodynamic vs. Kinetic Control: Sometimes, reactions can produce multiple products, where one is favored kinetically (formed faster) and the other is favored thermodynamically (more stable). The reaction conditions (temperature, time) determine which product is dominant.
-
Protecting Groups: In complex molecules, protecting groups may be necessary to selectively react with a specific functional group without affecting others.
Practicing Product Prediction: A Step-by-Step Approach
To confidently predict the major product of any reaction:
-
Identify the Functional Groups: Determine the functional groups present in the reactants.
-
Determine the Reaction Type: Classify the reaction as substitution, addition, elimination, etc.
-
Predict the Mechanism: Determine the likely mechanism (SN1, SN2, E1, E2, etc.).
-
Consider Stereochemistry: If stereocenters are involved, predict the stereochemistry of the product(s).
-
Apply Relevant Rules: Apply rules like Markovnikov's rule, Zaitsev's rule, etc.
-
Consider Competing Reactions: Identify any competing reactions that might lead to different products.
-
Draw the Major Product: Draw the structure of the predicted major product, showing all stereochemistry and regiochemistry.
By systematically applying these steps, you'll significantly improve your ability to predict the major product of organic reactions. Remember that practice is key to mastering organic chemistry. Work through numerous examples, focusing on understanding the mechanisms and the underlying principles. This will build your intuition and allow you to quickly and accurately predict reaction outcomes. Don't be afraid to consult textbooks and online resources when needed. Continuous learning is vital for success in this field.
Latest Posts
Latest Posts
-
How The Federal Government Aligns Resources And Delivers Core Capabilities
May 09, 2025
-
Horizontal Integration Helped Me Monopolize The Oil Market
May 09, 2025
-
Identify A Lateral Projection Of A Vertebra
May 09, 2025
-
What Does Set And Coordinate Distribution Objectives Mean
May 09, 2025
-
Ups 8 Keys To Lifting And Lowering
May 09, 2025
Related Post
Thank you for visiting our website which covers about Draw The Major Product Of This Reaction. Ignore Inorganic Byproducts. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.