Each Period In The Periodic Table Corresponds To
Breaking News Today
Apr 05, 2025 · 6 min read
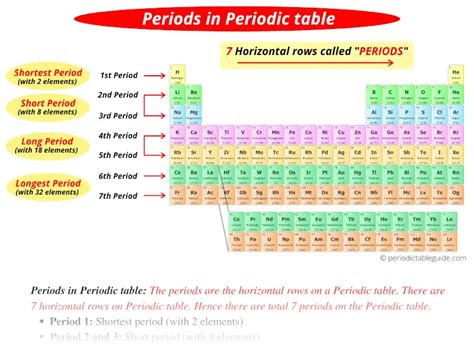
Table of Contents
Each Period in the Periodic Table Corresponds To: A Deep Dive into Electron Shells and Atomic Properties
The periodic table, that iconic grid of elements, is more than just a neatly organized list. It's a powerful tool that reveals fundamental relationships between atoms, predicting their properties and behaviors. One of the most crucial organizing principles is the period, each representing a distinct electron shell. Understanding what each period corresponds to is key to grasping the intricacies of chemistry and the predictable patterns in atomic structure.
Understanding Electron Shells and Periods
At the heart of the periodic table's organization lies the electron shell model. Atoms are composed of a nucleus containing protons and neutrons, surrounded by electrons orbiting in energy levels called shells. These shells are not physical orbits but rather regions of space where electrons are most likely to be found. Each shell can hold a specific maximum number of electrons.
- The first shell (n=1): Can hold a maximum of 2 electrons.
- The second shell (n=2): Can hold a maximum of 8 electrons.
- The third shell (n=3): Can hold a maximum of 18 electrons.
- The fourth shell (n=4) and beyond: The maximum number of electrons increases further, following more complex rules.
Each period in the periodic table corresponds to a principal quantum number (n), which dictates the energy level of the outermost electron shell being filled. As you move down the table, you add another shell. Therefore:
- Period 1: Elements in this period fill the first electron shell (n=1).
- Period 2: Elements in this period fill the second electron shell (n=2).
- Period 3: Elements in this period fill the third electron shell (n=3).
- Period 4 and beyond: The pattern continues, with each period corresponding to filling the next principal energy level.
This seemingly simple correspondence has profound implications for the properties of the elements. Let's explore each period in detail.
Period 1: The Simplest Elements
Period 1 contains only two elements: hydrogen (H) and helium (He). Both elements fill the first electron shell. Hydrogen has one electron in this shell, while helium has two, completely filling the shell. This complete shell explains helium's extraordinary inertness – it has no tendency to gain, lose, or share electrons.
Key characteristics of Period 1 elements:
- Small atomic radius: The single shell is very close to the nucleus.
- High ionization energy: It requires significant energy to remove an electron due to the strong attraction to the nucleus.
- High electronegativity (for hydrogen): Hydrogen strongly attracts electrons in a chemical bond.
Period 2: The Introduction of Subshells
Period 2 introduces the concept of subshells. While the first shell has only one subshell (s), the second shell has two: s and p. This means that period 2 elements fill the 2s and 2p subshells.
This period includes elements like lithium (Li), beryllium (Be), boron (B), carbon (C), nitrogen (N), oxygen (O), fluorine (F), and neon (Ne). The properties of these elements vary significantly depending on the number of electrons in the 2s and 2p subshells, leading to a range of chemical behaviors.
Key characteristics of Period 2 elements:
- Increased atomic radius compared to Period 1: The addition of a new shell increases the distance of outermost electrons from the nucleus.
- Lower ionization energy compared to Period 1: Electrons are less tightly bound to the nucleus.
- Varied electronegativity: Ranges from relatively low (lithium) to very high (fluorine).
- Formation of diverse chemical bonds: Elements exhibit a range of bonding capabilities, including covalent and ionic bonding.
Period 3: Expanding the Subshell Pattern
Period 3 follows a similar pattern, filling the 3s and 3p subshells. This period further expands the diversity of chemical properties, with elements like sodium (Na), magnesium (Mg), aluminum (Al), silicon (Si), phosphorus (P), sulfur (S), chlorine (Cl), and argon (Ar). The increasing number of electrons and the increasing distance from the nucleus create subtle yet significant shifts in atomic properties.
Key characteristics of Period 3 elements:
- Further increased atomic radius compared to Period 2.
- Lower ionization energy than Period 2.
- Varied electronegativity, but generally lower than Period 2 non-metals.
- Diverse bonding capabilities, with a larger emphasis on metallic bonding for the left side of the period.
Periods 4, 5, 6, and 7: Introducing d and f Orbitals and Increasing Complexity
Periods 4, 5, 6, and 7 introduce another layer of complexity. In addition to the s and p subshells, these periods involve the filling of the d subshells (transition metals) and the f subshells (lanthanides and actinides). The filling of these inner shells results in a more nuanced variation of properties, such as:
- Increased number of electrons: Leads to greater shielding effects, influencing the effective nuclear charge.
- Expanded range of oxidation states: Transition metals show a remarkable ability to exist in various oxidation states, contributing to their rich chemistry.
- Variable ionization energies: Shielding effects and electron configurations result in more irregular trends.
- Complex coordination chemistry: Transition metals form complex ions with ligands, creating diverse and intricate structures.
- Lanthanide and actinide contraction: The poor shielding effect of the f-electrons causes a decrease in atomic radius that is unexpected from the usual trends.
Period 4: Transition metals begin
Period 4 features the first row of transition metals, elements whose d orbitals are being filled. This leads to unique properties such as variable oxidation states, complex ion formation, and catalytic activity.
Period 5: Expanded Transition Metals and Similar Trends
Period 5 expands on the transition metal series and introduces similar trends as in period 4. The increased number of electrons introduces more subtle changes in properties compared to period 4.
Period 6: Lanthanides and Continued Transition Metal Series
Period 6 is remarkable for the inclusion of the lanthanides (rare earth elements), a series of elements where the 4f orbitals are being filled. This series displays similar chemical properties due to the similar electronic configurations.
Period 7: Actinides and the End of the Known Elements
Period 7 includes the actinides, another series of elements characterized by the filling of 5f orbitals. Many of the actinides are radioactive and synthetically produced.
Trends across Periods: A Summary
As we progress across a period from left to right:
- Atomic radius generally decreases: The increasing nuclear charge pulls the electrons closer to the nucleus.
- Ionization energy generally increases: It becomes increasingly difficult to remove an electron due to the stronger nuclear attraction.
- Electronegativity generally increases: The tendency to attract electrons in a bond increases.
- Metallic character generally decreases: Elements shift from metallic to non-metallic behavior.
Conclusion: The Periodic Table – A Powerful Tool for Understanding Atomic Structure
The periodic table's organization, especially its periods, provides a powerful framework for understanding the relationships between elements' properties and their electron configurations. By examining the correspondence between each period and the filling of electron shells, we can predict and explain a vast array of chemical behaviors, from the inertness of noble gases to the catalytic prowess of transition metals. The periodic table is not just a table; it is a map to the fundamental building blocks of matter, revealing the elegant order underlying the seemingly chaotic world of atoms. Further study into each period reveals even more nuanced details, making it a constant source of fascinating discovery for chemists and scientists alike. Understanding the periods is fundamental to understanding chemistry itself.
Latest Posts
Latest Posts
-
Unit 7 Progress Check Mcq Part C
Apr 05, 2025
-
Well Balanced People Are More Productive Employees
Apr 05, 2025
-
What Traumatic Event Changes The Seventh Mans Life
Apr 05, 2025
-
Alvaro Y Yo Nos Vamos A Casar Pronto
Apr 05, 2025
-
Accurate Interpretation Of A Prescription Helps To Ensure
Apr 05, 2025
Related Post
Thank you for visiting our website which covers about Each Period In The Periodic Table Corresponds To . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
