Enzyme Complexes That Break Down Protein Are Called _____.
Breaking News Today
Mar 19, 2025 · 6 min read
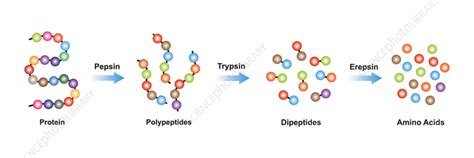
Table of Contents
Enzyme Complexes That Break Down Protein Are Called _____. Proteases: A Deep Dive into Protein Degradation
Enzyme complexes that break down proteins are called proteases, also known as peptidases or proteinases. These are a diverse group of enzymes crucial for numerous biological processes, from digestion and nutrient absorption to cellular regulation and immune response. Understanding their structure, function, and mechanisms is key to appreciating their pivotal role in maintaining life. This article will delve deep into the fascinating world of proteases, exploring their classifications, mechanisms of action, roles in various biological pathways, and clinical significance.
Understanding Proteases: The Masters of Protein Degradation
Proteins, the workhorses of the cell, are constantly being synthesized and degraded. This dynamic equilibrium is essential for maintaining cellular homeostasis and responding to internal and external stimuli. Proteases are the key players in the protein degradation process, cleaving peptide bonds that hold amino acid residues together. This breakdown releases individual amino acids, which can then be reused for protein synthesis or utilized for energy production.
The Diverse World of Protease Classifications
Proteases are classified in various ways, primarily based on their catalytic mechanisms and the nature of their active sites. These classifications aren't mutually exclusive; a protease can fall into multiple categories.
1. Catalytic Mechanism:
- Serine proteases: These enzymes utilize a serine residue in their active site for catalysis. Examples include trypsin, chymotrypsin (involved in digestion), and thrombin (crucial for blood clotting). They are characterized by a catalytic triad involving serine, histidine, and aspartate.
- Cysteine proteases: These employ a cysteine residue at their active site, often in the context of a catalytic dyad. Examples include caspases (involved in apoptosis or programmed cell death), cathepsins (lysosomal enzymes), and papain (found in papaya).
- Aspartic proteases: These utilize two aspartic acid residues in their active site for catalysis. Renin (involved in blood pressure regulation) and pepsin (involved in stomach acid digestion) are prominent examples.
- Metalloproteases: These proteases require a metal ion, usually zinc, for their catalytic activity. Matrix metalloproteinases (MMPs), which are involved in extracellular matrix remodeling, are a prime example. They play a critical role in tissue development, wound healing, and cancer metastasis.
- Threonine proteases: These utilize a threonine residue for catalysis. Examples include the proteasome, a large multi-subunit complex involved in the ubiquitin-proteasome system (UPS).
2. Substrate Specificity:
Proteases also exhibit varying degrees of substrate specificity. Some are highly specific, cleaving only certain peptide bonds, while others have broader specificity. This specificity is determined by the amino acid sequence surrounding the cleavage site and the architecture of the protease's active site.
Mechanisms of Protease Action: A Closer Look
The process of protein degradation by proteases typically involves several steps:
-
Recognition: The protease recognizes and binds to its target protein. This recognition can be influenced by factors such as the primary, secondary, tertiary, and quaternary structure of the protein, as well as post-translational modifications like phosphorylation or glycosylation.
-
Cleavage: Once bound, the protease cleaves the peptide bond, leading to the fragmentation of the protein. The exact mechanism of cleavage varies depending on the type of protease. For instance, serine proteases employ a mechanism involving the formation of a covalent intermediate between the substrate and the active site serine residue.
-
Release: After cleavage, the protease releases the resulting peptide fragments. The protease can then repeat the process, further degrading the protein or moving to a new substrate.
The Ubiquitin-Proteasome System: A Central Player in Regulated Protein Degradation
The ubiquitin-proteasome system (UPS) is a major pathway for regulated protein degradation in eukaryotic cells. It involves the tagging of target proteins with ubiquitin, a small regulatory protein, followed by degradation by the 26S proteasome, a large multi-subunit protease complex.
Key Components of the UPS:
- Ubiquitin-activating enzymes (E1): These enzymes activate ubiquitin, making it ready for conjugation.
- Ubiquitin-conjugating enzymes (E2): These enzymes transfer ubiquitin from E1 to E3.
- Ubiquitin ligases (E3): These enzymes recognize and bind to specific target proteins and catalyze the attachment of ubiquitin chains to them. The specificity of E3 ligases determines which proteins are targeted for degradation.
- 26S proteasome: This complex unfolds and degrades ubiquitinated proteins. It consists of a 20S core particle (CP), which contains the protease active sites, and two 19S regulatory particles (RPs), which recognize and unfold ubiquitinated proteins before feeding them into the CP.
Biological Roles of Proteases: A Broad Spectrum of Functions
Proteases play essential roles in a vast array of biological processes, including:
- Digestion: Proteases such as pepsin, trypsin, and chymotrypsin are crucial for breaking down dietary proteins into absorbable amino acids in the digestive system.
- Blood clotting: Thrombin, a serine protease, is essential for the formation of blood clots, preventing excessive bleeding.
- Immune response: Several proteases are involved in the immune system, including those that cleave complement proteins and antibodies, activating the immune cascade.
- Apoptosis (programmed cell death): Caspases, a family of cysteine proteases, play a pivotal role in apoptosis, a crucial process for development and eliminating damaged cells.
- Extracellular matrix remodeling: Matrix metalloproteinases (MMPs) are involved in breaking down and rebuilding the extracellular matrix, a process important for tissue development, wound healing, and angiogenesis (formation of new blood vessels).
- Protein quality control: The UPS plays a vital role in eliminating misfolded or damaged proteins, preventing their accumulation and potential toxicity.
- Signal transduction: Proteolytic cleavage of certain proteins can activate or inactivate signaling pathways, regulating cellular processes.
Clinical Significance of Proteases: Implications for Disease and Therapy
Dysregulation of protease activity is implicated in many diseases, including:
- Cancer: Abnormal activity of proteases like MMPs is often associated with tumor invasion, metastasis, and angiogenesis.
- Inflammatory diseases: Overactive protease activity contributes to inflammation in conditions such as rheumatoid arthritis and inflammatory bowel disease.
- Neurodegenerative diseases: Dysfunction of the UPS and proteolytic systems is implicated in neurodegenerative diseases like Alzheimer's disease and Parkinson's disease, due to the accumulation of misfolded proteins.
- Cardiovascular diseases: Proteases play a complex role in cardiovascular disease, impacting blood pressure regulation, blood clotting, and plaque formation.
Consequently, proteases have become important targets for drug development. Protease inhibitors are used to treat various diseases, including HIV/AIDS (targeting HIV protease), hypertension (targeting renin), and certain cancers (targeting MMPs).
The Future of Protease Research: Unfolding New Discoveries
The field of protease research is constantly evolving. Ongoing research is focused on:
- Discovering new proteases and understanding their functions: The human genome encodes a vast number of proteases, many of whose functions remain unknown.
- Developing more specific and effective protease inhibitors: This aims to minimize off-target effects and enhance therapeutic efficacy.
- Exploring the role of proteases in complex biological processes: Research continues to unravel the intricate roles proteases play in development, aging, and disease.
- Developing novel therapeutic strategies targeting protease activity: This includes the design of new drugs, gene therapies, and diagnostic tools.
Conclusion: Proteases – The Essential Orchestrators of Life
Proteases are indispensable enzymes orchestrating a myriad of cellular processes crucial for life. Their diverse functions and involvement in disease make them a focal point of extensive research. The continued exploration of their intricate mechanisms, roles, and therapeutic potential promises to unravel further insights into the fundamental processes of life and disease, leading to advancements in medicine and biotechnology. Further research into the specificity of proteases, their interactions with other cellular components, and the development of novel inhibitors will undoubtedly pave the way for more effective treatments for a wide range of diseases. This continued investigation into the multifaceted world of proteases solidifies their position as essential components of biological systems and vital targets for therapeutic intervention.
Latest Posts
Latest Posts
-
What Types Of Mollusks Have A Closed Circulatory System
Mar 19, 2025
-
Why Are Deserts Often Found Near Mountain Ranges
Mar 19, 2025
-
List Four Common Characteristics Of All Animals
Mar 19, 2025
-
A Compass Rose Was Part Of
Mar 19, 2025
-
Explain The Difference Between Physical Activity And Exercise
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Enzyme Complexes That Break Down Protein Are Called _____. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
