Explain Why Water Is A Polar Molecule
Breaking News Today
Mar 30, 2025 · 6 min read
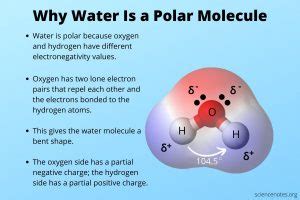
Table of Contents
Why Water is a Polar Molecule: A Deep Dive into the Science
Water. It's the lifeblood of our planet, essential for all known forms of life, and yet, its unique properties stem from a surprisingly simple, yet profound, characteristic: its polarity. Understanding why water is a polar molecule is crucial to grasping its remarkable behavior and its vital role in biological systems and the environment. This article will delve into the intricacies of water's polarity, exploring the underlying chemical bonds, molecular geometry, and resulting properties that make it so special.
The Foundation: Understanding Polarity
Before we dissect the polarity of water, let's establish a fundamental understanding of what "polar" means in the context of chemistry. A polar molecule is a molecule that possesses a net dipole moment – meaning it has a slightly positive end and a slightly negative end. This separation of charge arises from differences in electronegativity between the atoms within the molecule.
Electronegativity refers to an atom's ability to attract electrons in a chemical bond. Atoms with higher electronegativity pull electrons closer to themselves, creating a partial negative charge (δ-), while atoms with lower electronegativity have a partial positive charge (δ+). The greater the difference in electronegativity between two bonded atoms, the more polar the bond becomes.
The Structure of Water: A Bent Molecule
Water's chemical formula is H₂O, meaning each molecule consists of one oxygen atom and two hydrogen atoms. The oxygen atom forms covalent bonds with each hydrogen atom. A covalent bond involves the sharing of electrons between atoms. However, the sharing isn't always equal. This is where the electronegativity difference comes into play.
Oxygen is significantly more electronegative than hydrogen. This means that the oxygen atom attracts the shared electrons more strongly than the hydrogen atoms. This unequal sharing of electrons leads to a polar covalent bond, where the oxygen atom acquires a partial negative charge (δ-) and each hydrogen atom acquires a partial positive charge (δ+).
The molecule's geometry further contributes to its polarity. Water adopts a bent molecular geometry, due to the presence of two lone pairs of electrons on the oxygen atom. These lone pairs repel the bonding electron pairs, pushing the hydrogen atoms closer together and resulting in a bent, rather than linear, structure. This bent shape ensures that the partial positive charges of the hydrogen atoms and the partial negative charge of the oxygen atom don't cancel each other out. Instead, they create a net dipole moment, with the oxygen end being relatively negative and the hydrogen end relatively positive.
Visualizing the Polarity
Imagine a water molecule as a slightly bent "V" shape. The oxygen atom sits at the apex, carrying a partial negative charge. The two hydrogen atoms form the arms of the "V", each carrying a partial positive charge. This charge separation is what constitutes water's polarity. This asymmetry in charge distribution is key to many of water's extraordinary properties.
The Consequences of Water's Polarity: Remarkable Properties
The polarity of water is not just a chemical curiosity; it's the driving force behind many of its unique properties, which are essential for life as we know it.
1. High Boiling Point and Melting Point:
Compared to other molecules of similar size (like methane, CH₄), water has exceptionally high boiling and melting points. This is because the polar water molecules are strongly attracted to each other through hydrogen bonds. A hydrogen bond is a special type of intermolecular force that occurs between a hydrogen atom bonded to a highly electronegative atom (like oxygen) and another electronegative atom in a different molecule. These hydrogen bonds require significant energy to break, resulting in the high boiling and melting points.
2. Excellent Solvent:
Water's polarity makes it an excellent solvent, often referred to as the "universal solvent". Its polar nature allows it to dissolve many ionic compounds and polar molecules. The slightly negative oxygen end of a water molecule attracts the positive ions of a solute, while the slightly positive hydrogen ends attract the negative ions. This interaction effectively surrounds and separates the ions, dissolving the compound. This solvency is critical for biological processes, as many biological reactions take place in aqueous solutions.
3. High Surface Tension:
Water exhibits high surface tension due to the strong cohesive forces between its molecules caused by hydrogen bonding. These cohesive forces create a strong "skin" on the water's surface, allowing certain insects to walk on water. This property is also important in capillary action, the ability of water to move against gravity in narrow tubes, crucial for plant water transport.
4. High Specific Heat Capacity:
Water has a remarkably high specific heat capacity, meaning it can absorb a large amount of heat energy without a significant temperature increase. This property is vital for regulating temperature in living organisms and in the environment. Large bodies of water moderate temperature fluctuations, preventing drastic temperature changes.
5. Density Anomaly:
Water exhibits an unusual density anomaly: ice is less dense than liquid water. This is because the hydrogen bonds in ice create a relatively open, crystalline structure, resulting in a lower density. This property is critical for aquatic life, as the ice layer on the surface insulates the water below, preventing it from freezing solid.
Beyond the Basics: Implications for Life and the Environment
The consequences of water's polarity extend far beyond its physical properties. Its role in biological processes is paramount. Water acts as a medium for biochemical reactions, transporting nutrients and removing waste products. It participates directly in many metabolic reactions, such as hydrolysis and dehydration synthesis. The unique properties of water are essential for maintaining the structure and function of cells and organisms.
In the environment, water's polarity affects weather patterns, erosion, and the distribution of nutrients. Its ability to dissolve various substances contributes to the cycling of essential elements and the formation of solutions in soil and aquatic systems. The high specific heat capacity of water moderates global temperatures, while its role in the water cycle is vital for the distribution of freshwater resources.
Conclusion: A Remarkable Molecule
Water's polarity is not merely a textbook definition; it's the fundamental basis for a vast array of its extraordinary properties. Understanding this polarity unveils the intricate mechanisms behind water's critical role in sustaining life and shaping our planet. From its ability to act as a universal solvent to its high specific heat capacity and unique density anomaly, water's polar nature is a testament to the power of molecular structure and its profound influence on the world around us. Its properties continue to inspire scientific inquiry and highlight the importance of understanding the fundamental building blocks of our natural world. The seemingly simple water molecule is, in reality, a complex and remarkable substance whose significance cannot be overstated.
Latest Posts
Latest Posts
-
Apprentices Typically Receive A Pay Increase After Each
Apr 01, 2025
-
The Presidents Challenge Program Is A Fitness Evaluation Designed For
Apr 01, 2025
-
Amoeba Sisters Video Recap Answers Dna Replication
Apr 01, 2025
-
Which Of These Is Underconsumed In The United States
Apr 01, 2025
-
In The 1960s Margaret Crane Was Working As A
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Explain Why Water Is A Polar Molecule . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
