First Thirty Elements Of The Periodic Table
Breaking News Today
Mar 25, 2025 · 7 min read
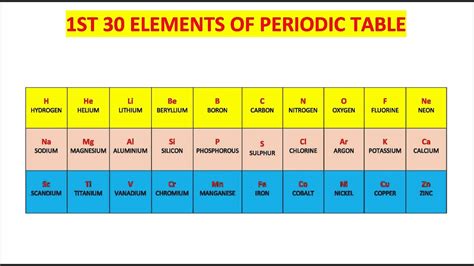
Table of Contents
The First Thirty Elements: A Deep Dive into the Building Blocks of Matter
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic number and recurring chemical properties. Understanding the first thirty elements is crucial for grasping fundamental chemical principles and their applications in various fields. This comprehensive guide delves into the properties, characteristics, and real-world uses of these foundational elements, providing a robust understanding of their significance in the world around us.
Understanding the Periodic Table's Structure
Before diving into the specifics of the first thirty elements, let's briefly review the periodic table's structure. Elements are arranged in rows (periods) and columns (groups). Periods represent the number of electron shells, while groups indicate the number of valence electrons – electrons in the outermost shell that determine the element's reactivity. Elements within the same group exhibit similar chemical behavior. The table is divided into metals, nonmetals, and metalloids, reflecting their physical and chemical properties.
The First Thirty Elements: A Detailed Exploration
We'll explore the first thirty elements (Hydrogen to Zinc) grouped by their periodic table families or characteristics.
Group 1: Alkali Metals (Except Hydrogen)
-
Lithium (Li): A soft, silvery-white metal. It's used in rechargeable batteries, ceramics, and lubricating greases due to its light weight and reactivity. Its compounds are also used in psychiatric medications.
-
Sodium (Na): Essential for human life, sodium is abundant in salt (NaCl). It's used extensively in the chemical industry, food processing (as a preservative and flavor enhancer), and street lighting (sodium vapor lamps). Its high reactivity makes it crucial in various chemical reactions.
-
Potassium (K): Another vital element for human health, potassium plays a critical role in maintaining fluid balance and nerve function. It's found in fertilizers to promote plant growth and is used in various industrial applications.
-
Rubidium (Rb) & Caesium (Cs): These elements are less common than sodium and potassium but share similar chemical properties. They are used in specialized applications like atomic clocks and certain types of lasers.
Group 2: Alkaline Earth Metals
-
Beryllium (Be): A lightweight, strong metal used in aerospace applications and high-performance alloys due to its exceptional stiffness and low density. It's toxic, requiring careful handling.
-
Magnesium (Mg): A lightweight metal widely used in alloys for automotive parts, structural materials, and electronics. It's also crucial in photosynthesis in plants and essential for human health. Magnesium hydroxide is used as an antacid.
-
Calcium (Ca): Essential for human bone structure and many biological processes. Calcium carbonate (limestone) is used in construction, while calcium sulfate (gypsum) finds application in plaster and drywall.
-
Strontium (Sr): Used in fireworks to produce brilliant red flames. Some strontium compounds are used in specialized applications like refining sugar.
-
Barium (Ba): Used in drilling muds in oil exploration and in specialized glass.
Group 13: Boron Group
-
Boron (B): A metalloid used in semiconductors, glass fibers (borosilicate glass), and detergents. Its compounds are important in agriculture as fertilizers and pesticides.
-
Aluminum (Al): The most abundant metal in the Earth's crust. Widely used in packaging, construction, and transportation due to its light weight, corrosion resistance, and recyclability.
-
Gallium (Ga): Used in semiconductors, LEDs, and high-temperature thermometers due to its unusual melting point and electrical properties.
Group 14: Carbon Group
-
Carbon (C): The basis of all organic life. Exists in various forms (diamond, graphite, fullerenes) with drastically different properties. It’s used in steelmaking, fuels, and countless other applications.
-
Silicon (Si): Used extensively in the electronics industry to manufacture semiconductors and integrated circuits. It's also found in glass, ceramics, and silicones.
-
Germanium (Ge): Used in semiconductors, particularly in high-frequency applications, and in fiber optics.
Group 15: Pnictogens
-
Nitrogen (N): A crucial component of the atmosphere and amino acids. Used in fertilizers, explosives, and in the production of ammonia.
-
Phosphorus (P): Essential for living organisms, involved in energy transfer and DNA structure. Found in fertilizers and detergents.
-
Arsenic (As): Toxic in most forms. Historically used in pesticides and wood preservatives but is being phased out due to its toxicity. Limited use in semiconductors.
-
Antimony (Sb): Used in lead-acid batteries, flame retardants, and certain alloys.
Group 16: Chalcogens
-
Oxygen (O): Essential for respiration and combustion. A vital component of water and numerous compounds.
-
Sulfur (S): Used in the production of sulfuric acid, a crucial industrial chemical, and in vulcanizing rubber.
-
Selenium (Se): An essential trace element used in some nutritional supplements. Used in photocopiers and solar cells.
Group 17: Halogens
-
Fluorine (F): The most reactive element. Used in fluoridated water to prevent tooth decay and in refrigerants (although many such refrigerants are being phased out).
-
Chlorine (Cl): Used in water purification, as a disinfectant, and in the production of various chemicals.
-
Bromine (Br): Used in flame retardants, water treatment, and in the production of certain dyes.
Group 18: Noble Gases
-
Helium (He): Used in balloons, cryogenics, and magnetic resonance imaging (MRI) machines. Its inertness makes it valuable in various applications.
-
Neon (Ne): Used in neon lights and some lasers.
-
Argon (Ar): Used as an inert atmosphere in welding and in incandescent light bulbs.
Transition Metals (Groups 3-12)
-
Scandium (Sc): Used in high-intensity lighting and in certain alloys.
-
Titanium (Ti): A strong, lightweight metal with high corrosion resistance, used in aerospace, medical implants, and sports equipment.
-
Vanadium (V): Used in steel alloys to improve strength and toughness.
-
Chromium (Cr): Used in chrome plating, stainless steel, and pigments due to its corrosion resistance and attractive appearance.
-
Manganese (Mn): An essential element for plants and animals. Used in steel alloys to improve strength and hardness.
-
Iron (Fe): The most abundant element in the Earth's crust. Crucial for oxygen transport in the blood (hemoglobin). Used extensively in steel and cast iron.
-
Cobalt (Co): Used in magnets, alloys, and as a catalyst in some industrial processes.
-
Nickel (Ni): Used in stainless steel, nickel-cadmium batteries, and as a catalyst.
-
Copper (Cu): An excellent conductor of electricity, used in electrical wiring, plumbing, and alloys like brass and bronze.
-
Zinc (Zn): Used in galvanization to protect iron and steel from corrosion. Also used in batteries and alloys.
Applications and Significance of the First Thirty Elements
The first thirty elements are not merely abstract chemical entities; they form the foundation of modern technology and are crucial for sustaining life on Earth. Their diverse properties and applications are integral to many aspects of our lives:
-
Technology: Silicon is paramount in electronics, while titanium and aluminum are vital in aerospace and construction. Copper is essential for electrical systems, and many elements play crucial roles in the manufacturing of numerous materials.
-
Biology and Medicine: Elements like oxygen, carbon, hydrogen, nitrogen, calcium, phosphorus, potassium, sodium, and many trace elements are essential for all life forms. Many elements find direct applications in medicine – from treating illnesses to constructing medical implants.
-
Industry: The chemical industry heavily relies on these elements in the production of various materials, from plastics to fertilizers. Their uses span numerous other sectors, including energy, construction, and transportation.
-
Everyday Life: The impact of these elements extends to everyday life. They are found in our food, our homes, our clothes, and the countless products we use daily.
Environmental Considerations
While these elements are vital, their extraction, processing, and use can have environmental impacts. Sustainable practices are essential to minimize these effects. For instance, responsible mining practices and recycling programs are crucial for reducing the environmental footprint associated with obtaining and utilizing these valuable resources. Proper disposal of materials containing potentially hazardous elements, like arsenic and lead, is vital for protecting both human health and the environment.
Conclusion: The Enduring Importance of the First Thirty Elements
The first thirty elements represent a crucial subset of the periodic table, offering a fundamental understanding of chemical principles and their widespread applications. From the life-sustaining roles of elements like oxygen and carbon to the technological marvels enabled by silicon and titanium, these elements underpin modern society. Understanding their properties and potential environmental impacts is vital for responsible scientific advancement and maintaining a sustainable future. Further exploration of their interactions and properties will continue to yield new insights and technologies, solidifying their enduring importance in shaping our world.
Latest Posts
Latest Posts
-
Kangaroo Is To Marsupial As Ballad Is To
Mar 27, 2025
-
Management Of A Medical Unit Hesi Case Study
Mar 27, 2025
-
Relias Progressive Care Rn Assessment A Answers
Mar 27, 2025
-
How Did He Actually Slow Scientific Progress In Western Europe
Mar 27, 2025
-
Ati Rn Maternal Newborn Online Practice 2023 A
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about First Thirty Elements Of The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
