How Many Grams Are In 1.946 Moles Of Nacl
Breaking News Today
Jun 08, 2025 · 4 min read
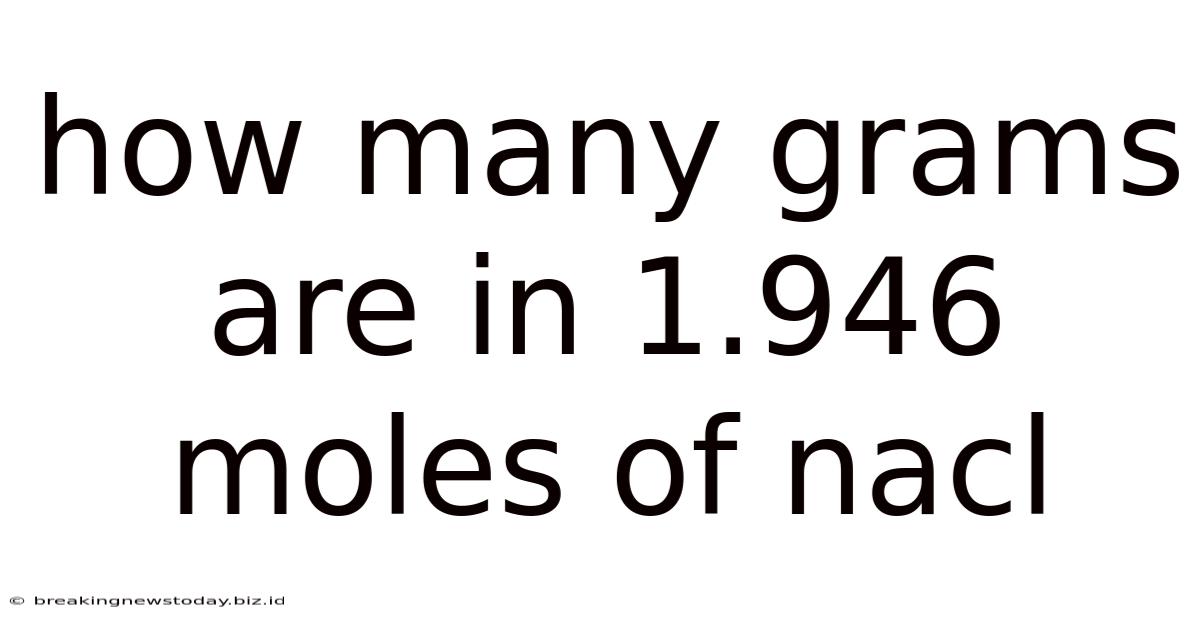
Table of Contents
How Many Grams Are in 1.946 Moles of NaCl? A Comprehensive Guide to Mole Conversions
Understanding mole conversions is fundamental in chemistry. This article will comprehensively guide you through calculating the grams in 1.946 moles of NaCl (sodium chloride), commonly known as table salt. We'll delve into the underlying principles, explore practical applications, and offer tips to master this essential chemistry concept.
Understanding Moles and Molar Mass
Before we tackle the calculation, let's clarify the key concepts:
1. Mole (mol): The mole is a fundamental unit in chemistry, representing a specific number of particles (atoms, molecules, ions, etc.). This number is known as Avogadro's number, approximately 6.022 x 10<sup>23</sup>. One mole of any substance contains Avogadro's number of particles.
2. Molar Mass (g/mol): Molar mass represents the mass of one mole of a substance. It's expressed in grams per mole (g/mol). To calculate the molar mass of a compound, you need to sum the atomic masses (in grams per mole) of all the atoms present in the compound's chemical formula.
Calculating the Molar Mass of NaCl
NaCl, sodium chloride, is an ionic compound composed of one sodium (Na) atom and one chlorine (Cl) atom. To find its molar mass, we add the atomic masses of Na and Cl:
- Atomic mass of Na: Approximately 22.99 g/mol
- Atomic mass of Cl: Approximately 35.45 g/mol
Molar mass of NaCl = Atomic mass of Na + Atomic mass of Cl = 22.99 g/mol + 35.45 g/mol = 58.44 g/mol
Converting Moles to Grams: The Formula
The fundamental formula for converting moles to grams is:
Mass (in grams) = Number of moles × Molar mass (g/mol)
This formula allows us to convert the given number of moles of a substance into its corresponding mass in grams.
Calculating the Mass of 1.946 Moles of NaCl
Now, we can apply this formula to our problem:
- Number of moles: 1.946 mol
- Molar mass of NaCl: 58.44 g/mol
Mass (in grams) = 1.946 mol × 58.44 g/mol = 113.76 g (approximately)
Therefore, there are approximately 113.76 grams in 1.946 moles of NaCl.
Practical Applications of Mole Conversions
The ability to convert between moles and grams is crucial in various chemical contexts:
-
Stoichiometry: In stoichiometric calculations, we use mole ratios to determine the amounts of reactants and products in chemical reactions. Converting moles to grams allows us to relate these amounts to measurable masses.
-
Solution Preparation: When preparing solutions of a specific concentration (e.g., molarity), we need to accurately weigh out the required mass of solute. Mole conversions are essential for this process.
-
Analytical Chemistry: Analytical techniques often involve determining the amount of a substance in a sample. The results are frequently expressed in moles, which then need to be converted to grams for practical interpretation.
-
Industrial Chemistry: Large-scale chemical processes require precise control of reactant amounts. Mole conversions are vital for ensuring efficient and safe operation.
Error Analysis and Significant Figures
It's important to consider the accuracy of our calculations. The atomic masses we used are approximations. The number of significant figures in our final answer should reflect the precision of our input values. In this case, 1.946 moles has four significant figures, and 58.44 g/mol also has four significant figures. Therefore, our final answer, 113.76 g, should also have four significant figures.
Further Exploration of Mole Concepts
Beyond simple mole-to-gram conversions, understanding moles opens up a wider world of chemical calculations:
-
Mole-to-particle conversions: Using Avogadro's number, you can convert moles to the number of atoms, molecules, or ions.
-
Molar volume: At standard temperature and pressure (STP), one mole of any ideal gas occupies a volume of approximately 22.4 liters.
-
Percent Composition: Determining the percentage by mass of each element in a compound involves using molar mass.
-
Empirical and Molecular Formulas: Mole conversions are essential for determining the empirical and molecular formulas of compounds from experimental data.
Tips for Mastering Mole Conversions
-
Practice regularly: The more you practice mole conversions, the more comfortable you'll become with the process.
-
Use a systematic approach: Always write out the given information, the formula, and your calculations clearly and step-by-step.
-
Check your units: Ensure that your units cancel out correctly throughout your calculations.
-
Use a periodic table: Always have a periodic table handy to look up atomic masses.
-
Understand the concept: Don't just memorize the formula; understand the underlying principles of moles and molar mass.
Conclusion
Converting moles to grams is a fundamental skill in chemistry. By understanding the concepts of moles, molar mass, and the conversion formula, you can accurately determine the mass of a substance given its number of moles. This skill is essential for various applications in stoichiometry, solution preparation, analytical chemistry, and industrial processes. Through consistent practice and a solid grasp of the underlying principles, you can master this crucial aspect of chemistry. Remember to always pay attention to significant figures and units for accurate and reliable results. This comprehensive guide provides a strong foundation for tackling more complex chemical calculations involving moles and molar mass.
Latest Posts
Latest Posts
-
Draw The Lewis Structure For The Tellurium Tetrabromide Molecule
Jun 08, 2025
-
I Am A Rhombus I Have Perpendicular Sides
Jun 08, 2025
-
Bioflix Activity Population Ecology Types Of Population Growth
Jun 08, 2025
-
The Museum Turned Out To Be
Jun 08, 2025
-
A Formal Classification Challenge Begins With Which Of The Following
Jun 08, 2025
Related Post
Thank you for visiting our website which covers about How Many Grams Are In 1.946 Moles Of Nacl . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.