How Many Valence Electrons Does A Carbon Atom Have
Breaking News Today
Mar 14, 2025 · 5 min read
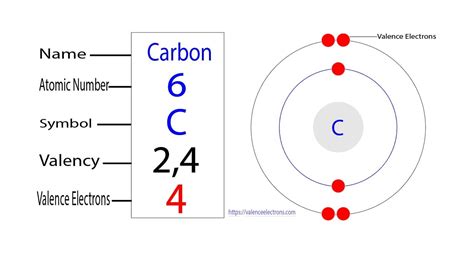
Table of Contents
How Many Valence Electrons Does a Carbon Atom Have? A Deep Dive into Carbon's Chemistry
Carbon, the backbone of life and a cornerstone of countless materials, boasts a unique electronic structure that underpins its remarkable versatility. Understanding the number of valence electrons in a carbon atom is key to grasping its incredible ability to form diverse and complex molecules. This article will delve into the intricacies of carbon's electronic configuration, exploring not only the simple answer to the question of valence electrons but also the far-reaching consequences of this seemingly small detail.
Understanding Valence Electrons
Before focusing specifically on carbon, let's establish a foundational understanding of valence electrons. Valence electrons are the electrons located in the outermost shell of an atom. These are the electrons most involved in chemical bonding because they are the furthest from the nucleus and experience the weakest electrostatic attraction. The number of valence electrons directly dictates an atom's reactivity and the types of bonds it can form. It determines the atom's capacity to gain, lose, or share electrons to achieve a stable electron configuration, often following the octet rule (eight electrons in the outer shell for stability).
Carbon's Electronic Configuration: Unveiling the Secret to its Versatility
Carbon's atomic number is 6, meaning it possesses six protons and six electrons in a neutral atom. To determine the number of valence electrons, we need to examine its electronic configuration. Using the Aufbau principle and Hund's rule, we can distribute these six electrons across the available energy levels:
- 1s² 2s² 2p²
This configuration reveals that:
- The first shell (n=1) contains two electrons in the 1s orbital.
- The second shell (n=2) contains four electrons: two in the 2s orbital and two in the 2p orbitals.
Therefore, carbon has four valence electrons. These four valence electrons reside in the second energy level (n=2) and are responsible for carbon's extensive bonding capabilities.
The Significance of Four Valence Electrons
The presence of four valence electrons is what makes carbon so unique and essential in the world of chemistry and biology. This number allows carbon to form strong covalent bonds with a variety of atoms, including itself. This ability to form stable bonds with other carbon atoms is the foundation of organic chemistry and the existence of complex organic molecules.
Carbon's Bonding Prowess: A Multifaceted Approach
Carbon's four valence electrons enable it to exhibit a wide range of bonding behaviors:
1. Single Bonds: The Foundation of Stability
Carbon can form four single covalent bonds, sharing one electron with each of four other atoms. This is exemplified in methane (CH₄), where carbon shares one electron with each of four hydrogen atoms, achieving a stable octet configuration for both carbon and hydrogen. This single-bond structure forms the basis of many organic molecules and is highly stable.
2. Double Bonds: Enhanced Strength and Rigidity
Carbon can also form double bonds, sharing two electrons with another atom. This type of bond is shorter and stronger than a single bond. Ethylene (C₂H₄) is a classic example, where each carbon atom forms a double bond with the other carbon atom and two single bonds with hydrogen atoms. Double bonds contribute to the rigidity of many molecules.
3. Triple Bonds: The Strongest Link
Carbon can even form triple bonds, sharing three electrons with another atom. These bonds are even shorter and stronger than double bonds. Acetylene (C₂H₂) provides a clear example, where each carbon atom forms a triple bond with the other carbon atom and a single bond with a hydrogen atom. Triple bonds often lead to linear structures.
4. Diverse Bonding Partners: Carbon's Versatility
The remarkable aspect of carbon's bonding is its ability to bond with a diverse range of atoms, including hydrogen, oxygen, nitrogen, sulfur, phosphorus, and halogens. This diversity contributes to the vast array of organic compounds found in nature and synthesized in laboratories.
Carbon's Role in Organic Chemistry and Biology
The unique properties stemming from its four valence electrons make carbon the central element in organic chemistry and the foundation of life itself.
1. The Building Blocks of Life: Organic Molecules
Carbon's ability to form long chains, branched structures, and rings is crucial for the formation of large and complex organic molecules, including:
- Carbohydrates: Sugars and starches, providing energy for living organisms.
- Lipids: Fats and oils, serving as energy storage and structural components.
- Proteins: Essential for a myriad of biological functions, including enzymes and structural support.
- Nucleic acids: DNA and RNA, carrying genetic information.
These complex molecules wouldn't exist without carbon's unique bonding capabilities.
2. The Variety of Organic Compounds: An Astonishing Range
The number of possible organic compounds is practically limitless due to carbon's ability to form diverse structures and bond with a wide range of elements. This staggering diversity is a direct consequence of carbon's four valence electrons. From simple hydrocarbons to complex biomolecules, carbon is the essential element driving this extraordinary chemical variety.
3. Materials Science and Technology: Carbon's Contribution
Beyond its biological significance, carbon is also vital in materials science and technology. Its diverse forms, such as diamond, graphite, and fullerenes, possess unique properties that find applications in various fields:
- Diamond: Known for its hardness and brilliance, used in cutting tools and jewelry.
- Graphite: A soft, conductive material used in pencils and batteries.
- Fullerenes: Unique cage-like structures with potential applications in nanotechnology and medicine.
These diverse applications highlight the versatility of carbon and its profound impact on human endeavors.
Conclusion: The Four Valence Electrons That Shaped the World
The seemingly simple answer—four—to the question of how many valence electrons carbon possesses belies the immense complexity and significance of this element. These four electrons are the key to carbon's remarkable ability to form an almost infinite variety of molecules, driving the richness of organic chemistry, sustaining life itself, and underpinning countless technological advancements. Understanding carbon's valence electrons provides a foundational understanding of its crucial role in shaping the world around us, from the smallest biological molecules to the largest technological innovations. Further exploration into carbon's chemistry continues to uncover new and exciting possibilities, reaffirming its enduring importance in science and technology.
Latest Posts
Latest Posts
-
Earthquakes Occur At Transform Boundaries Responses True True False
Mar 14, 2025
-
What Does The National Minimum Drinking Age Act Prohibit
Mar 14, 2025
-
How Should Trash And Recyclables Be Stored
Mar 14, 2025
-
A New Office Building Would Be An Example Of This
Mar 14, 2025
-
What Year Was The Royal Period In Georgia
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Does A Carbon Atom Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
