Humane Endpoint Criteria Define The Conditions Under Which
Breaking News Today
Mar 22, 2025 · 6 min read
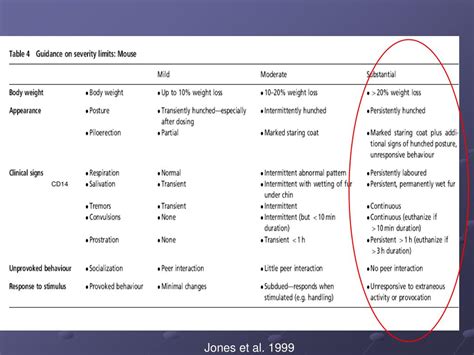
Table of Contents
Humane Endpoint Criteria: Defining the Conditions Under Which Animal Studies Must Stop
Animal research plays a vital role in advancing scientific knowledge and developing life-saving treatments. However, it's crucial to conduct such research ethically and responsibly, minimizing animal suffering. A cornerstone of responsible animal research is the establishment and strict adherence to humane endpoint criteria. These criteria define the specific conditions under which an animal study must be terminated to prevent unnecessary pain, distress, or suffering. This article delves deep into humane endpoint criteria, exploring their importance, components, development, implementation, and ongoing challenges.
The Importance of Humane Endpoint Criteria
The ethical use of animals in research necessitates a commitment to minimizing their suffering. Humane endpoint criteria are not simply guidelines; they are essential safeguards ensuring that animals aren't subjected to prolonged or unnecessary pain. Their implementation reflects a commitment to the 3Rs principle: Replacement, Reduction, and Refinement. Humane endpoints directly contribute to Refinement by ensuring procedures are modified to minimize pain and distress. By setting clear limits on acceptable suffering, they prevent prolonged experiments that might yield minimal scientific benefit.
The absence of well-defined humane endpoint criteria can lead to:
- Unnecessary suffering: Animals may endure prolonged pain, distress, and discomfort without intervention.
- Inconsistent treatment: Different researchers might have varying interpretations of what constitutes unacceptable suffering, leading to inconsistencies in animal care.
- Compromised research results: Prolonged suffering can impact animal behavior and physiology, potentially confounding research results.
- Ethical violations: Failure to adhere to humane endpoint criteria constitutes a breach of ethical guidelines and regulations.
- Reputational damage: Institutions and researchers failing to prioritize animal welfare risk severe reputational consequences.
Defining the Components of Humane Endpoint Criteria
Humane endpoint criteria are not a one-size-fits-all solution. They must be study-specific, tailored to the particular species, experimental procedures, and potential adverse effects. However, several common components typically form the basis of these criteria:
1. Pre-defined Clinical Signs: These are observable indicators of pain, distress, or suffering, such as:
- Weight loss: Significant and sustained weight loss exceeding a pre-defined percentage of baseline body weight.
- Dehydration: Visible signs of dehydration, including sunken eyes, decreased skin turgor, and lethargy.
- Lethargy and decreased activity: Marked reduction in spontaneous activity and responsiveness.
- Changes in behavior: Aggression, self-mutilation, unusual vocalizations, or changes in social interaction.
- Abnormal respiratory patterns: Rapid, labored, or shallow breathing.
- Changes in appetite and food consumption: Significant decrease or complete absence of food intake.
- Changes in body temperature: Significant deviations from the normal physiological range.
- Presence of tumors or lesions: The development of large or rapidly growing tumors that cause significant discomfort.
- Signs of infection or sepsis: Elevated temperature, lethargy, and other signs of infection.
- Skin or coat changes: Ulcerations, excessive grooming, or changes in coat condition.
2. Severity and Duration of Clinical Signs: It's not just the presence of clinical signs but also their severity and duration that dictate the application of humane endpoints. A single mild clinical sign may not warrant euthanasia, but the persistence or worsening of symptoms would necessitate intervention.
3. Quantitative Measures: In some cases, objective quantitative measures can supplement clinical observations. This might include:
- Blood chemistry analysis: Monitoring blood parameters to detect organ dysfunction or metabolic disturbances.
- Body temperature monitoring: Regular monitoring of body temperature to detect significant fluctuations.
- Pain scales: Utilizing validated pain assessment scales specific to the species and experimental model.
4. Survival Time: For certain studies, particularly those involving tumor models, a pre-determined survival time may be included as part of the humane endpoint criteria. This ensures that animals aren't subjected to prolonged suffering associated with terminal illness.
5. Specific procedures-related adverse effects: Certain research procedures carry unique risks. For example, surgical procedures might require humane endpoints for specific post-operative complications, such as excessive bleeding, wound infection, or failure to thrive.
Development and Implementation of Humane Endpoint Criteria
The development of robust humane endpoint criteria requires careful consideration and collaboration among various stakeholders:
- Veterinary staff: Veterinarians play a critical role, possessing the expertise to assess animal health and identify signs of pain and distress.
- Researchers: Scientists conducting the study must understand the potential adverse effects of their procedures and incorporate these into the humane endpoint criteria.
- Ethical review boards (ERBs or IACUCs): ERBs review and approve research protocols, including the proposed humane endpoint criteria, ensuring they are adequate and ethically sound.
The implementation of humane endpoint criteria requires:
- Regular monitoring of animals: Animals should be monitored frequently by trained personnel for any signs of pain, distress, or suffering.
- Detailed record-keeping: All observations and interventions related to animal welfare must be meticulously documented.
- Training of personnel: All personnel involved in animal research must be trained in recognizing and responding to signs of distress and implementing humane endpoint criteria.
- Clear communication protocols: Effective communication channels must be in place to ensure timely intervention when humane endpoints are triggered.
Challenges and Ongoing Developments in Humane Endpoint Criteria
Despite the critical importance of humane endpoint criteria, several challenges remain:
1. Subjectivity in Assessing Animal Distress: Assessing animal suffering can be subjective, especially with non-vocal species. Developing objective, validated methods for assessing pain and distress in various animal models remains an active area of research.
2. Species-Specific Differences: What constitutes acceptable suffering varies across species, demanding species-specific criteria. Developing universally applicable criteria is difficult, requiring deep knowledge of the specific species' behavior and physiology.
3. Balancing Scientific Goals with Animal Welfare: Researchers must balance the need to obtain robust scientific data with the ethical obligation to minimize animal suffering. Defining humane endpoints often involves a delicate balancing act, requiring careful consideration of the potential benefits and risks of the research.
4. Lack of Standardized Guidelines: While many guidelines exist, inconsistencies in the application and interpretation of humane endpoint criteria across institutions and countries pose a significant challenge. Standardization and harmonization of guidelines could improve consistency and ethical oversight.
5. Advancing Technologies: Technological advancements are leading to the development of novel approaches for monitoring animal welfare, such as advanced imaging techniques, biosensors, and artificial intelligence. Integrating these technologies could enhance the accuracy and objectivity of humane endpoint assessments.
The Future of Humane Endpoint Criteria
The continuous refinement and improvement of humane endpoint criteria are crucial for ensuring the responsible and ethical conduct of animal research. Future developments will likely focus on:
- Development of objective measures of pain and distress: This involves developing and validating new tools and techniques for accurately assessing animal suffering across different species.
- Increased standardization of guidelines: Harmonizing guidelines and protocols across different institutions and countries will enhance consistency in the application of humane endpoints.
- Integration of advanced technologies: Utilizing emerging technologies to monitor animal welfare in real-time will improve the accuracy and timeliness of interventions.
- Enhanced training and education: Providing comprehensive training to researchers and animal care personnel on recognizing and responding to signs of distress is paramount.
- Promoting open communication and collaboration: Encouraging open sharing of best practices and collaborative efforts across institutions will advance the development and implementation of humane endpoint criteria.
In conclusion, humane endpoint criteria are indispensable for ensuring the ethical and humane treatment of animals in research. While challenges remain, ongoing efforts to refine and standardize these criteria, along with advancements in technology and a heightened commitment to animal welfare, are paving the way for a more compassionate and responsible approach to animal research. The commitment to these criteria is not merely a regulatory requirement but a fundamental ethical imperative reflecting the respect and value we place on animal life.
Latest Posts
Latest Posts
-
Which Pain Condition Requires The Administration Of Opioid Drugs Quizlet
Mar 23, 2025
-
Which Type Of Briefing Is Delivered To Individual Resources Quizlet
Mar 23, 2025
-
How Is The President Of The United States Elected Quizlet
Mar 23, 2025
-
A Potential Risk Factor For Breast Cancer Includes Quizlet
Mar 23, 2025
-
Corporate Taxes Are A Type Of Quizlet
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about Humane Endpoint Criteria Define The Conditions Under Which . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
