If Neutral Atoms Become Positive Ions They
Breaking News Today
Mar 21, 2025 · 6 min read
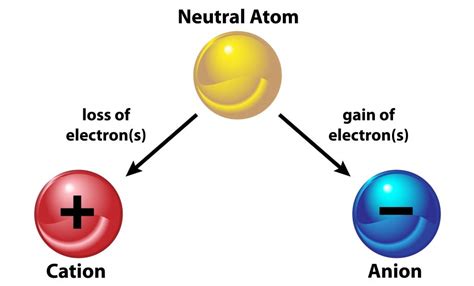
Table of Contents
If Neutral Atoms Become Positive Ions, What Happens? A Deep Dive into Ionization
When a neutral atom sheds one or more of its electrons, it transforms into a positively charged ion, a process known as ionization. This seemingly simple change has profound consequences, impacting the atom's properties, reactivity, and behavior in various environments. This article will explore the intricacies of ionization, delving into its mechanisms, effects on atomic structure and properties, and its significant role in diverse scientific fields.
The Mechanism of Ionization
Ionization fundamentally involves disrupting the delicate balance between the positive charge of the nucleus and the negative charge of the electrons. In a neutral atom, the number of protons (positive charge) in the nucleus equals the number of electrons (negative charge) orbiting it. This equilibrium creates a stable, electrically neutral entity. However, several mechanisms can overcome this stability and lead to ionization:
1. Collisional Ionization: A High-Energy Encounter
This is a common mechanism where a neutral atom collides with a high-energy particle, such as another atom, an ion, or a photon. The energy transferred during the collision can exceed the ionization energy—the minimum energy required to remove an electron from the atom. This excess energy ejects an electron, leaving behind a positively charged ion. The likelihood of collisional ionization significantly increases with higher kinetic energies of the colliding particles and the number of collisions.
2. Photoionization: Light's Ionizing Power
Photons, or particles of light, possess energy proportional to their frequency. High-frequency photons, such as those in ultraviolet (UV) or X-ray radiation, carry sufficient energy to ionize atoms. When a photon interacts with an atom, its energy can be absorbed by an electron, providing it with enough energy to escape the atom's electrostatic attraction. This process is vital in various phenomena, including the formation of the ionosphere and the operation of photomultiplier tubes. The effectiveness of photoionization depends heavily on the photon's energy and the atom's ionization energy.
3. Chemical Ionization: Electron Transfer in Chemical Reactions
In certain chemical reactions, electrons can be transferred from one atom to another. If an atom loses an electron to a more electronegative atom, it becomes a positive ion. This is often seen in redox (reduction-oxidation) reactions where one species is oxidized (loses electrons) while another is reduced (gains electrons). The relative electronegativities of the reacting atoms play a crucial role in determining the direction of electron transfer and the formation of positive ions.
4. Thermal Ionization: Heat as an Ionization Agent
High temperatures can also induce ionization. At elevated temperatures, atoms gain significant kinetic energy, leading to more frequent and energetic collisions. These collisions can supply the energy needed to overcome the atom's ionization energy, leading to the ejection of electrons and the formation of positive ions. Thermal ionization is critical in plasmas, where a significant fraction of atoms are ionized due to high temperatures. This process is central to technologies like plasma torches and fusion reactors.
Effects of Ionization on Atomic Structure and Properties
The loss of electrons significantly alters an atom's characteristics. Several key changes occur:
-
Net Positive Charge: The most apparent change is the acquisition of a net positive charge, equal to the number of electrons lost. For instance, losing one electron results in a +1 ion, losing two electrons produces a +2 ion, and so on.
-
Reduced Atomic Radius: With fewer electrons to shield the positive nuclear charge, the remaining electrons are drawn closer to the nucleus, resulting in a smaller atomic radius. The effective nuclear charge increases, leading to a stronger attraction between the nucleus and the remaining electrons.
-
Altered Chemical Reactivity: The ionization process drastically alters an atom's chemical reactivity. Positive ions readily participate in chemical reactions, often acting as Lewis acids, accepting electron pairs from other species. Their positive charge makes them highly attractive to negatively charged ions or molecules.
-
Changes in Spectral Lines: The energy levels of electrons in an atom are quantized. Ionization changes the electronic configuration, leading to a distinct change in the atom's emission and absorption spectrum. This change in spectral lines is a powerful tool for identifying ions and studying their properties.
-
Magnetic Properties: The presence of unpaired electrons in the ion can lead to paramagnetism – a weak attraction towards a magnetic field. The magnetic properties of ions are significant in various applications, including MRI technology and magnetic resonance imaging.
Significance of Ionization in Various Fields
The process of ionization holds immense significance across numerous scientific and technological domains:
1. Spectroscopy: Unraveling Atomic Structures
The analysis of atomic spectra, particularly the changes in spectral lines after ionization, provides invaluable insights into the electronic structure of atoms and ions. Spectroscopic techniques help determine the energy levels of electrons, ionization energies, and other fundamental atomic properties. This is critical for understanding the behavior of matter at the atomic level.
2. Mass Spectrometry: Identifying and Quantifying Ions
Mass spectrometry is an analytical technique used to identify and quantify ions based on their mass-to-charge ratio. This technique is widely used in various fields, including biochemistry, environmental science, and materials science, to analyze the composition of samples, identify unknown compounds, and quantify specific molecules.
3. Plasma Physics: Harnessing the Power of Ionized Gases
Plasmas, often referred to as the "fourth state of matter," are partially or fully ionized gases. Ionization processes play a central role in creating and sustaining plasmas, which are utilized in numerous applications, such as plasma displays, plasma etching in semiconductor manufacturing, and controlled fusion research. Understanding ionization mechanisms is crucial for controlling and manipulating plasma properties.
4. Atmospheric Chemistry: Understanding Atmospheric Processes
Ionization processes significantly influence the chemistry of the Earth's atmosphere. Photoionization by solar radiation creates ions in the upper atmosphere, leading to the formation of the ionosphere, which plays a vital role in radio wave propagation and satellite communication. Ion-molecule reactions in the atmosphere drive many important chemical cycles, including the ozone cycle and the formation of acid rain.
5. Astrophysics: Studying Celestial Objects
Ionization processes are central to understanding the behavior of stars and other celestial objects. High temperatures within stars lead to extensive ionization, resulting in plasmas that govern stellar structure and evolution. The analysis of stellar spectra, which reveal the presence and abundance of various ions, provides essential information about the composition and physical conditions of stars. Understanding ionization is key to analyzing nebulas, galaxies, and other celestial structures.
6. Medical Applications: Radiation Therapy and Imaging
Ionizing radiation, such as X-rays and gamma rays, is used in medical imaging techniques like X-ray radiography and computed tomography (CT) scans. Controlled ionization is also used in radiation therapy to treat cancer by targeting and damaging cancerous cells. Understanding the interaction of ionizing radiation with biological tissues is critical for optimizing the effectiveness and safety of these medical applications.
Conclusion: The Far-Reaching Impact of Ionization
The transformation of a neutral atom into a positive ion, though seemingly a simple process, has far-reaching consequences across numerous scientific and technological disciplines. From fundamental atomic physics and spectroscopy to the complexities of atmospheric chemistry and astrophysics, the study of ionization mechanisms and the properties of ions remains crucial for understanding the behavior of matter at the atomic and molecular levels and for developing innovative technologies. The continued exploration of ionization processes will undoubtedly lead to further advancements in various fields, providing deeper insights into the fundamental laws of nature and enabling the development of cutting-edge applications.
Latest Posts
Latest Posts
-
How Did Reza Pahlavi Differ From Ayatollah Khomeini
Mar 28, 2025
-
Natural Concepts Are Mental Groupings Created Naturally Through Our
Mar 28, 2025
-
The Gift Of The Magi Answer Key
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about If Neutral Atoms Become Positive Ions They . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
