If You Add More Enzyme The Reaction Will
Breaking News Today
Mar 18, 2025 · 5 min read
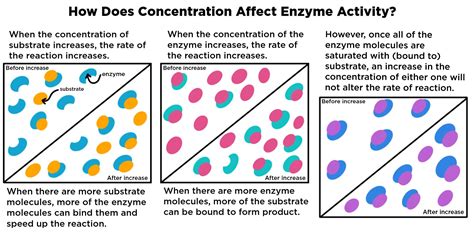
Table of Contents
If You Add More Enzyme, the Reaction Will... Speed Up (But Only to a Point)
Enzymes are biological catalysts that dramatically speed up the rate of virtually all chemical reactions within cells. Understanding how enzyme concentration affects reaction rates is fundamental to grasping many biological processes, from digestion to DNA replication. This article delves into the intricate relationship between enzyme concentration and reaction speed, exploring the limitations and nuances involved.
The Enzyme-Substrate Complex: The Heart of the Matter
Before diving into the effects of increased enzyme concentration, let's briefly review the basic mechanism of enzyme action. Enzymes work by binding to specific molecules called substrates. This binding forms an enzyme-substrate complex, creating a microenvironment conducive to the reaction. The enzyme facilitates the reaction, often by lowering the activation energy – the energy required to initiate the reaction. Once the reaction is complete, the products are released, and the enzyme is free to bind to another substrate molecule.
Think of it like a lock and key: the enzyme (lock) is highly specific to its substrate (key). Only the correctly shaped substrate can fit into the enzyme's active site, initiating the reaction.
The Impact of Increasing Enzyme Concentration
The rate of an enzyme-catalyzed reaction is directly proportional to the enzyme concentration, provided there is sufficient substrate available. This is because, with more enzymes, more enzyme-substrate complexes can form simultaneously. Consequently, more substrate molecules are converted into products per unit of time.
In simpler terms: More enzymes mean more reactions happening concurrently, leading to a faster overall reaction rate.
Visualizing the Effect: The Rate vs. Enzyme Concentration Graph
The relationship between enzyme concentration and reaction rate is typically depicted using a graph. The x-axis represents the enzyme concentration, and the y-axis represents the reaction rate. Initially, the graph shows a linear relationship: as enzyme concentration increases, the reaction rate increases proportionally. This is the region where substrate is plentiful, and the enzyme is the limiting factor.
However, this linear relationship doesn't continue indefinitely. Eventually, the curve flattens out, reaching a plateau. This indicates that adding more enzyme no longer significantly increases the reaction rate.
Reaching Saturation: The Point of Diminishing Returns
The plateau in the graph represents the saturation point. At this point, all available substrate molecules are already bound to enzymes. Adding more enzymes won't increase the reaction rate because there are no more free substrate molecules to bind to. The enzymes are essentially waiting for substrate molecules to become available, leading to a maximal reaction rate. The enzymes are saturated with substrate, and the rate is limited by the availability of the substrate, not the enzymes.
This saturation point highlights a crucial aspect of enzyme kinetics: enzyme concentration alone doesn't dictate the reaction rate indefinitely. Other factors, notably substrate concentration, play a critical role in determining the overall speed of the reaction.
Other Factors Influencing Reaction Rate
While enzyme concentration is a significant factor, other variables significantly impact the rate of enzyme-catalyzed reactions:
1. Substrate Concentration:
As mentioned earlier, substrate concentration directly impacts the reaction rate. At low substrate concentrations, the reaction rate is directly proportional to substrate concentration. However, as substrate concentration increases, the reaction rate eventually plateaus, reaching Vmax (maximum velocity). This is because all enzyme active sites are occupied, and further increases in substrate don't lead to faster reaction rates.
2. Temperature:
Temperature affects enzyme activity significantly. Generally, increasing temperature initially increases reaction rate by increasing the kinetic energy of molecules, leading to more frequent collisions between enzymes and substrates. However, beyond a certain optimal temperature, high temperatures denature the enzyme, causing it to lose its three-dimensional structure and function, drastically reducing the reaction rate.
3. pH:
Enzymes have optimal pH ranges within which they function most efficiently. Deviations from this optimal pH can alter the enzyme's shape and activity, affecting the reaction rate. Extreme pH values can denature the enzyme, completely inhibiting its catalytic function.
4. Inhibitors:
Inhibitors are molecules that bind to enzymes and reduce their activity. Competitive inhibitors compete with substrates for the enzyme's active site, while non-competitive inhibitors bind to a different site on the enzyme, altering its shape and reducing its activity. The presence of inhibitors drastically reduces the reaction rate, even with high enzyme concentrations.
5. Activators:
Conversely, activators are molecules that enhance enzyme activity. They can bind to the enzyme, altering its shape to make it more effective in binding substrates and catalyzing reactions. Activators can increase the reaction rate even at lower enzyme concentrations.
6. Enzyme Cofactors:
Many enzymes require cofactors (metal ions or organic molecules) for their activity. The absence of these cofactors can significantly decrease or even completely inhibit the enzyme's function and therefore the reaction rate.
Practical Applications and Examples
Understanding the relationship between enzyme concentration and reaction rate has many practical applications in various fields:
-
Industrial Biotechnology: In industries utilizing enzymes for biocatalysis (e.g., food processing, biofuel production, pharmaceuticals), optimizing enzyme concentration is crucial for maximizing product yield and efficiency.
-
Medical Diagnostics: Enzyme activity assays are widely used in medical diagnostics to detect and monitor various diseases. Accurate measurement of enzyme activity requires controlling enzyme concentration and other relevant factors.
-
Agricultural Science: Understanding enzyme kinetics is essential for optimizing crop yields by managing factors that influence the activity of crucial plant enzymes involved in nutrient uptake and metabolism.
-
Environmental Science: Enzyme activity assays can be employed to assess the health of ecosystems and the impact of pollutants on environmental processes.
Conclusion: A Complex Interplay
While adding more enzyme generally increases the reaction rate, it's crucial to remember this relationship is not unlimited. The reaction rate will eventually plateau due to substrate saturation. Furthermore, other factors – substrate concentration, temperature, pH, inhibitors, activators and cofactors – significantly influence the overall reaction speed. Therefore, understanding the intricate interplay of these factors is essential for accurately predicting and manipulating the rates of enzyme-catalyzed reactions in various biological and industrial contexts. A comprehensive understanding of enzyme kinetics allows for optimizing reaction conditions and achieving desired outcomes in diverse applications. This knowledge is fundamental to many scientific disciplines and technological advancements.
Latest Posts
Latest Posts
-
Which Of The Following Is An Example Of Removable Media
Mar 18, 2025
-
Which Is The Graph Of Linear Inequality 2y X 2
Mar 18, 2025
-
Hot Glass Looks The Same As Cold Glass
Mar 18, 2025
-
Why Did Many Immigrants Settle In The Cities
Mar 18, 2025
-
Many Of The Progressive Reformers Were
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about If You Add More Enzyme The Reaction Will . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
