In The Molecule Bri Which Atom Is The Negative Pole
Breaking News Today
Mar 31, 2025 · 5 min read
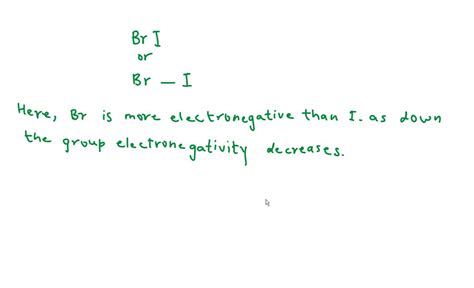
Table of Contents
In the BRI Molecule, Which Atom is the Negative Pole? Understanding Molecular Polarity
Determining the negative pole in a molecule like BRI requires understanding molecular polarity and the electronegativity of its constituent atoms. While "BRI" isn't a recognized standard chemical abbreviation, let's assume it represents a hypothetical molecule for the purpose of illustrating the principles involved in identifying the negative pole. We'll explore concepts applicable to any molecule to understand how to determine the negative pole.
Understanding Electronegativity and Polarity
The concept of a "negative pole" in a molecule arises from polarity. A molecule is polar if it possesses a dipole moment, meaning there's an uneven distribution of electron density. This uneven distribution occurs due to differences in electronegativity among the atoms within the molecule.
Electronegativity is the ability of an atom to attract electrons towards itself in a chemical bond. Elements on the right side of the periodic table (excluding noble gases) generally have higher electronegativity than those on the left. Oxygen (O), nitrogen (N), fluorine (F), and chlorine (Cl) are particularly electronegative.
When atoms with differing electronegativities bond, the more electronegative atom pulls the shared electrons closer to itself. This creates a partial negative charge (δ-) on the more electronegative atom and a partial positive charge (δ+) on the less electronegative atom. This difference in charge distribution forms the dipole moment.
Identifying the Negative Pole in a Hypothetical BRI Molecule
Let's assume "BRI" represents a molecule composed of three hypothetical atoms: B, R, and I. To determine the negative pole, we need information about the electronegativity of B, R, and I. Since these are not standard chemical symbols, we'll need to make some assumptions.
Scenario 1: B is least electronegative, I is most electronegative.
Let's assume B has the lowest electronegativity, followed by R, and I has the highest. In this case, the I atom would attract electrons most strongly, creating a partial negative charge (δ-) on the I atom. The negative pole of the BRI molecule would be located at or near the I atom.
Scenario 2: Different Bonding Arrangements
The location of the negative pole also depends on the molecular geometry (shape) of the BRI molecule. If the molecule is linear (B-R-I), the negative pole will still be near I. However, if it's bent or has a more complex structure, the dipole moments of individual bonds could cancel each other out to some degree, resulting in a less pronounced or even zero overall dipole moment (a nonpolar molecule).
Scenario 3: Multiple Electronegative Atoms
If the molecule contains multiple electronegative atoms, the negative pole would be located near the atom with the highest electronegativity or the region where the combined effects of several electronegative atoms create the largest partial negative charge. Consider the molecule water (H₂O). Oxygen is more electronegative than hydrogen. The two O-H bonds create a bent geometry with a net dipole moment, resulting in a negative pole near the oxygen atom.
Factors Influencing Molecular Polarity
Several factors influence a molecule's polarity beyond the simple electronegativity difference:
- Bond polarity: This refers to the uneven distribution of electrons within an individual bond. Greater electronegativity difference leads to higher bond polarity.
- Molecular geometry: The three-dimensional arrangement of atoms determines how individual bond dipoles combine to create the overall molecular dipole moment. Symmetrical molecules may have zero dipole moment even with polar bonds.
- Lone pairs of electrons: Lone pairs of electrons on the central atom can contribute to molecular polarity. They influence the electron distribution, potentially leading to an overall dipole moment.
- Resonance: In some molecules, electron delocalization due to resonance can affect the electron distribution and hence the polarity.
Practical Examples: Understanding Polarity in Known Molecules
Let's look at some real-world examples to further solidify our understanding:
- Water (H₂O): Oxygen is more electronegative than hydrogen, and the bent molecular geometry ensures a net dipole moment, with the negative pole near the oxygen atom.
- Carbon dioxide (CO₂): Although oxygen is more electronegative than carbon, the linear geometry results in the bond dipoles canceling each other, making CO₂ a nonpolar molecule.
- Ammonia (NH₃): Nitrogen is more electronegative than hydrogen, and the pyramidal geometry leads to a net dipole moment with the negative pole near the nitrogen atom.
- Methane (CH₄): While carbon is slightly more electronegative than hydrogen, the tetrahedral geometry results in bond dipoles cancelling, making methane nonpolar.
Advanced Techniques for Determining Molecular Polarity
For complex molecules, determining polarity may require more advanced computational techniques like:
- Density Functional Theory (DFT): A quantum mechanical method used to calculate the electron distribution and determine molecular properties, including dipole moments.
- Molecular dynamics simulations: These simulations can model the behavior of molecules over time, providing insight into the dynamic aspects of their polarity.
Conclusion: Determining the Negative Pole
Determining the negative pole in any molecule, including our hypothetical BRI, relies on a comprehensive understanding of electronegativity, molecular geometry, and the interplay of other factors influencing electron distribution. While a simple comparison of electronegativity can provide a preliminary estimate, a deeper analysis accounting for molecular structure is crucial for accurately identifying the negative pole and the overall polarity of the molecule. The principles described here, however, provide a solid framework for approaching this problem in any molecule, regardless of its complexity. Remember that if you are working with a real molecule, consult the appropriate chemical literature for the correct electronegativity values and established molecular geometry.
Latest Posts
Latest Posts
-
The Results Of Can Lead To Changes In Scientific Knowledge
Apr 02, 2025
-
File Plan Rules Include But Are Not Limited To
Apr 02, 2025
-
The Data Component Of An Information System Is
Apr 02, 2025
-
Florida Real Estate Exam Questions And Answers Pdf
Apr 02, 2025
-
Which Of The Following Statements Applies To All Driving Emergency
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about In The Molecule Bri Which Atom Is The Negative Pole . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
