What 2 Subatomic Particles Make Up The Nucleus
Breaking News Today
Mar 31, 2025 · 6 min read
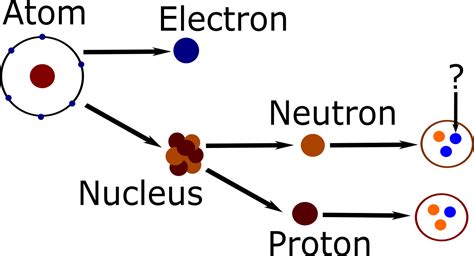
Table of Contents
What Two Subatomic Particles Make Up the Nucleus? A Deep Dive into Atomic Structure
The atom, once considered the fundamental building block of matter, has revealed itself to be a complex and fascinating microcosm of particles and forces. Understanding its structure is key to grasping the nature of matter itself. A central question in atomic physics concerns the nucleus: what two subatomic particles make it up? The answer is simple yet profound: protons and neutrons. However, a simple answer requires a much deeper explanation to fully appreciate the intricacies involved. This article delves into the properties of protons and neutrons, their interactions within the nucleus, and the broader implications of this fundamental structure.
Protons: The Positively Charged Guardians
Protons, one of the two crucial components of the atomic nucleus, carry a positive electrical charge, equal in magnitude but opposite in sign to the charge of an electron. This positive charge is crucial for the overall stability of the atom. The number of protons in an atom's nucleus determines its atomic number, which uniquely identifies the element. For example, hydrogen (H) has one proton, helium (He) has two, and uranium (U) has 92. This number is fundamental because it dictates the element's chemical properties and its place on the periodic table.
Key Properties of Protons:
- Charge: +1 (elementary charge)
- Mass: Approximately 1.6726 × 10⁻²⁷ kg (approximately 1836 times the mass of an electron)
- Spin: ½ (a quantum property related to intrinsic angular momentum)
- Composition: Composed of three quarks: two up quarks and one down quark. This quark composition contributes to the proton's overall charge and mass.
Neutrons: The Neutral Stabilizers
Neutrons, the other primary constituent of the nucleus, as their name suggests, carry no net electrical charge. Their role is essential in nuclear stability. While protons repel each other due to their positive charges, neutrons help to overcome this electrostatic repulsion and bind the nucleus together through the strong nuclear force. The number of neutrons in an atom's nucleus, along with the number of protons, determines the atomic mass number.
Key Properties of Neutrons:
- Charge: 0
- Mass: Approximately 1.6749 × 10⁻²⁷ kg (slightly larger than the mass of a proton)
- Spin: ½
- Composition: Composed of three quarks: one up quark and two down quarks. This composition results in a net neutral charge.
The Strong Nuclear Force: The Glue That Holds the Nucleus Together
The sheer proximity of positively charged protons within the tiny space of the nucleus should, according to classical electromagnetism, result in immediate repulsion and disintegration. However, the nucleus remains stable due to the strong nuclear force. This force is one of the four fundamental forces in nature, and it's significantly stronger than the electromagnetic force at short distances (within the nucleus). It's responsible for binding protons and neutrons together, overcoming the electrostatic repulsion between protons.
Understanding the Strong Force:
The strong force isn't a simple attraction; it's a complex interaction mediated by particles called gluons. Gluons are massless particles that carry the strong force between quarks. The quarks within protons and neutrons are constantly exchanging gluons, creating a dynamic and powerful binding effect. This interaction is crucial for maintaining the stability of the atomic nucleus, which in turn dictates the stability of matter itself.
Isotopes: Variations in Neutron Number
While the number of protons defines the element, the number of neutrons can vary. Atoms of the same element with different numbers of neutrons are called isotopes. For example, carbon-12 (¹²C) has six protons and six neutrons, while carbon-14 (¹⁴C) has six protons and eight neutrons. Most elements exist as a mixture of isotopes. Some isotopes are stable, while others are radioactive, undergoing decay processes that transform them into different elements. This radioactive decay is a consequence of the balance (or imbalance) between the strong nuclear force and other forces within the nucleus.
Nuclear Stability and the Neutron-to-Proton Ratio
The stability of an atomic nucleus is heavily influenced by the ratio of neutrons to protons. For lighter elements, a roughly equal ratio is often observed for stable isotopes. However, as the atomic number increases, the number of neutrons required for stability tends to exceed the number of protons. This is because the strong nuclear force has a limited range, and as the number of protons increases, the repulsive electromagnetic forces become more significant. The extra neutrons help to increase the strength of the strong nuclear force, thus stabilizing the nucleus. When this ratio deviates significantly from the optimal range, the nucleus becomes unstable and prone to radioactive decay.
Beyond Protons and Neutrons: The Quark Model
While protons and neutrons were initially thought to be fundamental particles, further research revealed they are composed of even smaller constituents called quarks. This discovery revolutionized our understanding of subatomic particles and led to the development of the Standard Model of particle physics.
Quarks: The Fundamental Building Blocks
Quarks are fundamental particles that interact via the strong force. There are six types, or "flavors," of quarks: up, down, charm, strange, top, and bottom. Protons and neutrons are composed of only the up and down quarks. The specific combination of quarks determines the particle's properties, such as charge and mass.
The Role of the Nucleus in Chemistry and Physics
The nucleus plays a pivotal role in both chemistry and physics. In chemistry, the number of protons (atomic number) determines the element's chemical behavior and its interactions with other elements. The arrangement of electrons surrounding the nucleus dictates the chemical bonds that form, influencing the properties of molecules and materials.
In physics, the nucleus is the site of nuclear reactions, including nuclear fission and fusion. These reactions release enormous amounts of energy, as seen in nuclear power plants and nuclear weapons. The study of nuclear physics has led to advancements in medicine, materials science, and energy production.
Further Explorations: Nuclear Models and Research
Scientists have developed various models to describe the structure and behavior of the nucleus, including the liquid drop model, the shell model, and the collective model. These models help explain various nuclear phenomena, such as nuclear binding energy, nuclear reactions, and radioactive decay. Ongoing research continues to refine our understanding of nuclear physics, exploring topics such as exotic nuclei, superheavy elements, and the properties of the strong nuclear force. The quest to understand the fundamental forces and particles that govern the universe continues, with the nucleus remaining a central focus of scientific inquiry.
Conclusion: A Foundation of Matter
The simple answer – protons and neutrons – belies the incredible complexity of the atomic nucleus. The interactions between these particles, governed by the strong nuclear force and mediated by gluons, are fundamental to the structure and stability of matter. Understanding the composition and behavior of the nucleus is crucial for advancing our knowledge in physics, chemistry, and related fields, unlocking possibilities for new technologies and deeper insights into the universe around us. The ongoing exploration of nuclear physics continues to reveal new layers of complexity and deepen our appreciation for the elegance and power of the natural world.
Latest Posts
Latest Posts
-
The Continuum Model Of Abnormality Demonstrates That
Apr 02, 2025
-
An Announcement Is What Type Of Communication
Apr 02, 2025
-
Name The Area Pictured In The Hootsuite Mobile App
Apr 02, 2025
-
The Process Of Adapting Borrowed Cultural Traits
Apr 02, 2025
-
Act Vocabulary Crossword Puzzle 1 Answer Key
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What 2 Subatomic Particles Make Up The Nucleus . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
