What Determines How Organic Molecules Will Look And Behave
Breaking News Today
Mar 21, 2025 · 6 min read
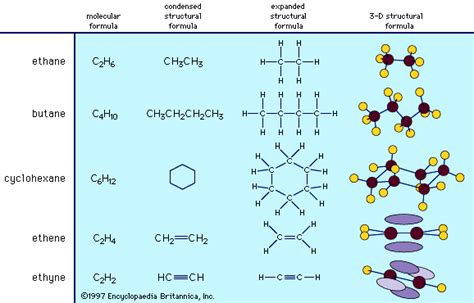
Table of Contents
What Determines How Organic Molecules Will Look and Behave?
The fascinating world of organic chemistry revolves around carbon-based molecules and their myriad properties. Understanding how these molecules look and behave is crucial across various scientific disciplines, from medicine and materials science to environmental science and food technology. This behavior isn't random; it's dictated by a complex interplay of factors, primarily stemming from the unique properties of carbon itself and the way it bonds with other atoms.
The Central Role of Carbon
Carbon's exceptional ability to form diverse molecules is rooted in its electronic structure. With four valence electrons, carbon can form four strong covalent bonds. This tetravalency allows for the creation of long chains, branched structures, rings, and complex three-dimensional frameworks. No other element exhibits this versatility to the same extent. This is the fundamental reason why millions of organic compounds exist, far exceeding the number of inorganic compounds.
Carbon's Bonding Prowess:
- Single Bonds: Carbon can form single covalent bonds (σ bonds) with various atoms, including hydrogen, oxygen, nitrogen, sulfur, halogens, and even other carbon atoms. These bonds are relatively strong and stable.
- Double Bonds: Carbon can also form double bonds (one σ bond and one π bond) resulting in planar geometry around the carbon atoms involved. This double bond introduces rigidity and restricts rotation around the bond axis.
- Triple Bonds: Triple bonds (one σ bond and two π bonds) are even stronger and shorter than double bonds. They create linear geometry and exhibit restricted rotation.
- Bond Length and Strength: The length and strength of carbon-carbon bonds vary depending on the bond order (single, double, or triple). Triple bonds are the shortest and strongest, followed by double bonds, and then single bonds. This difference significantly affects molecular properties.
Factors Influencing Molecular Structure and Behavior
The appearance and behavior of an organic molecule are determined by a complex interplay of several factors, acting together in a sophisticated dance:
1. Molecular Formula:
The molecular formula provides a basic inventory of atoms present in a molecule (e.g., C₂H₆ for ethane). While it tells us the types and numbers of atoms, it doesn't reveal the arrangement of these atoms, which critically impacts the molecule's properties. Isomers, compounds with the same molecular formula but different structures, perfectly illustrate this point.
2. Structural Formula:
The structural formula shows how atoms are bonded to each other. This is crucial as it determines the molecule's shape and ultimately its properties. Different structural isomers (e.g., butane and isobutane) have the same molecular formula (C₄H₁₀) but vastly different physical and chemical properties due to their distinct arrangements.
3. Isomerism:
Isomerism is a cornerstone of organic chemistry. It encompasses various types, each impacting molecular behavior in specific ways:
- Structural Isomerism: This involves different arrangements of atoms in a molecule. Examples include chain isomerism (different carbon chain lengths), positional isomerism (different positions of functional groups), and functional group isomerism (different functional groups).
- Stereoisomerism: This involves molecules with the same connectivity but different spatial arrangements. It includes geometrical isomerism (cis-trans or E-Z isomerism) and optical isomerism (enantiomers and diastereomers). Stereoisomers often exhibit significantly different biological activities, highlighting the critical role of 3D structure.
4. Functional Groups:
Functional groups are specific groups of atoms within a molecule that determine its chemical reactivity. These groups, such as hydroxyl (-OH), carbonyl (C=O), carboxyl (-COOH), amino (-NH₂), and others, dictate how a molecule will interact with other molecules, influencing its properties like boiling point, melting point, solubility, and reactivity. The presence and position of functional groups are key determinants of a molecule's behavior.
5. Intermolecular Forces:
The forces between molecules also significantly influence their behavior. These forces, which are weaker than the covalent bonds within a molecule, include:
- Van der Waals forces: These are weak, temporary attractions between molecules due to temporary fluctuations in electron distribution. They are present in all molecules.
- Dipole-dipole interactions: These occur between polar molecules possessing permanent dipoles. The partially positive end of one molecule attracts the partially negative end of another.
- Hydrogen bonding: This is a particularly strong type of dipole-dipole interaction involving hydrogen atoms bonded to highly electronegative atoms (like oxygen or nitrogen). Hydrogen bonding has a profound impact on the boiling points and other properties of molecules.
6. Molecular Size and Shape:
The overall size and three-dimensional shape of a molecule have a significant impact on its properties. Larger molecules generally have higher boiling points and melting points due to increased van der Waals forces. The shape also influences how molecules pack together in a solid or liquid, affecting their physical properties. Furthermore, the shape is critical for biological molecules, where specific shapes are essential for enzyme-substrate interactions and receptor binding.
7. Hybridization:
The concept of hybridization helps explain the bonding and geometry of carbon atoms. Carbon atoms can undergo sp, sp², and sp³ hybridization, leading to different bond angles and molecular shapes. For example, sp³ hybridization in methane (CH₄) results in a tetrahedral geometry, while sp² hybridization in ethene (C₂H₄) leads to a planar geometry. Hybridization significantly impacts molecular properties and reactivity.
8. Resonance:
In some molecules, the electrons are delocalized over several atoms, leading to resonance structures. This delocalization stabilizes the molecule and can influence its reactivity and other properties. Benzene, with its delocalized π electrons, is a classic example of resonance stabilization. The presence of resonance significantly alters bond lengths and bond energies, affecting the overall behavior of the molecule.
9. Conformational Isomerism:
Conformational isomers are different spatial arrangements of atoms in a molecule that can be interconverted by rotation around single bonds. These conformations have different energies, and the relative populations of different conformers depend on factors like temperature and steric hindrance. The most stable conformation is usually the one with the least steric strain. Steric effects, arising from the spatial arrangement of atoms, play a crucial role in determining molecular stability and reactivity.
10. Aromatic Compounds:
Aromatic compounds, characterized by a ring of atoms with delocalized π electrons, exhibit unique properties distinct from aliphatic (non-aromatic) compounds. The delocalized electrons contribute to their stability and unique reactivity patterns. Aromatic compounds are often more stable and less reactive than their aliphatic counterparts, due to the resonance stabilization afforded by the delocalized π electron cloud.
Predicting Molecular Behavior: A Multifaceted Approach
Predicting the exact behavior of an organic molecule requires integrating all the factors mentioned above. This is a complex task, often necessitating the use of sophisticated computational techniques and spectroscopic methods. However, a fundamental understanding of these factors provides a valuable framework for predicting general trends and properties.
For instance, knowing the presence of a polar functional group allows us to predict that the molecule will likely be soluble in polar solvents like water. Similarly, the presence of extensive π-conjugation can help predict increased UV-Vis absorption. The molecular size and shape can help estimate boiling points and melting points.
Conclusion: The Intricate Dance of Structure and Behavior
The structure and behavior of organic molecules are intrinsically linked. A molecule's shape, the types of bonds it possesses, the presence of functional groups, and the intermolecular forces it experiences all collectively determine its physical and chemical properties. By understanding these factors, we can begin to unravel the complexity of the organic world and harness the remarkable diversity of carbon-based molecules for various applications. The field continues to evolve, with new techniques and insights constantly deepening our understanding of this fundamental area of chemistry. From the development of new drugs and materials to the understanding of biological processes, the principles governing the structure and behavior of organic molecules remain central to scientific advancement.
Latest Posts
Latest Posts
-
Spyware Can Result In All The Following Except
Mar 28, 2025
-
Olga Lucia No Encuentra Su Cepillo Azul
Mar 28, 2025
-
What Was Napoleon Able To Accomplish During Peacetime
Mar 28, 2025
-
What Insect Symbolizes Both Death And Rebirth
Mar 28, 2025
-
Prevention Of The Spread Of Infections Begins And Ends With
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about What Determines How Organic Molecules Will Look And Behave . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
