What Is The Major Product Of The Following Reaction
Breaking News Today
Mar 19, 2025 · 6 min read
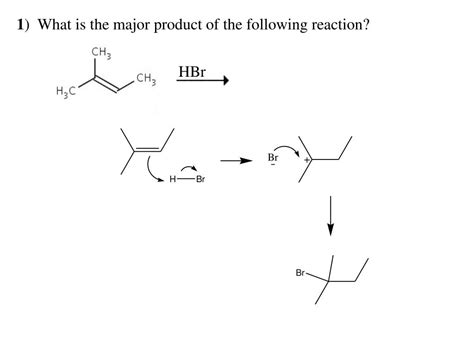
Table of Contents
Predicting the Major Product: A Deep Dive into Organic Reaction Mechanisms
Determining the major product of a chemical reaction is a cornerstone of organic chemistry. It requires a thorough understanding of reaction mechanisms, reaction kinetics, and the interplay of various factors influencing product selectivity. This article delves into the principles guiding prediction of major products, focusing on common reaction types and the nuances that dictate the outcome. We'll explore concepts like regioselectivity, stereoselectivity, and chemoselectivity, illustrating them with numerous examples. This comprehensive guide will empower you to confidently predict the major product for a wide array of organic reactions.
Understanding Reaction Mechanisms: The Foundation for Prediction
Before we delve into specific reactions, it's crucial to establish a strong understanding of reaction mechanisms. A reaction mechanism is a step-by-step description of how a reaction proceeds, detailing the bond-breaking and bond-forming events at the molecular level. Understanding the mechanism is key because it reveals the intermediates formed and the transition states traversed during the reaction. This information is essential for predicting the major product. Common mechanistic pathways include:
-
SN1 (Substitution Nucleophilic Unimolecular): This mechanism involves a two-step process: a rate-determining ionization step followed by a fast nucleophilic attack. SN1 reactions favor tertiary carbocations due to their greater stability. They typically lead to racemization at the reaction center.
-
SN2 (Substitution Nucleophilic Bimolecular): This mechanism involves a concerted, one-step process where the nucleophile attacks the substrate from the backside, leading to inversion of configuration at the stereocenter. SN2 reactions are favored by primary substrates and strong nucleophiles.
-
E1 (Elimination Unimolecular): This mechanism involves a two-step process: a rate-determining ionization step to form a carbocation followed by a base-induced proton abstraction. E1 reactions are favored by tertiary substrates and typically lead to a mixture of alkenes (Zaitsev's rule often predicts the major alkene).
-
E2 (Elimination Bimolecular): This mechanism involves a concerted, one-step process where the base abstracts a proton while simultaneously eliminating a leaving group. E2 reactions are favored by strong bases and often lead to specific alkene geometries depending on the substrate and base.
-
Addition Reactions (Electrophilic and Nucleophilic): These reactions involve the addition of a reagent across a multiple bond (e.g., double or triple bond). The regioselectivity and stereoselectivity of addition reactions are heavily influenced by the nature of the reactants and the reaction conditions. Markovnikov's rule often governs the regioselectivity of electrophilic addition to alkenes.
Factors Influencing Product Selectivity: A Multifaceted Approach
The major product of a reaction is not solely determined by the mechanism. Several other factors significantly influence the outcome:
-
Steric Hindrance: Bulky groups can hinder the approach of reactants, influencing the reaction rate and selectivity. This is particularly relevant in SN2 and E2 reactions.
-
Electronic Effects: Electron-donating and electron-withdrawing groups can influence the reactivity and stability of intermediates, thereby affecting product selectivity. Resonance stabilization plays a crucial role here.
-
Reaction Conditions: Temperature, solvent, concentration of reactants, and the presence of catalysts can all significantly impact the reaction pathway and the resulting product distribution.
-
Leaving Group Ability: The ability of a leaving group to depart from the substrate influences the rate of SN1, SN2, E1, and E2 reactions. Better leaving groups generally lead to faster reactions.
-
Nucleophile/Base Strength and Sterics: The strength and steric bulk of the nucleophile or base greatly influence the outcome, particularly in SN2 and E2 reactions. Stronger, less hindered nucleophiles/bases favor SN2 and E2 pathways respectively.
Predicting Major Products: Case Studies and Examples
Let's analyze some specific reaction types and illustrate how to predict the major product by considering the interplay of mechanistic principles and influencing factors:
1. SN1 Reaction of 2-bromo-2-methylpropane with methanol:
The tertiary carbocation formed is highly stable. Nucleophilic attack by methanol occurs from both sides, resulting in a racemic mixture of products: 2-methoxy-2-methylpropane.
2. SN2 Reaction of 1-bromopropane with sodium hydroxide:
The strong nucleophile (OH⁻) attacks the primary carbon from the backside, leading to inversion of configuration. The major product is 1-propanol.
3. E1 Reaction of 2-chloro-2-methylbutane:
The tertiary carbocation intermediate can undergo elimination to form two possible alkenes: 2-methyl-2-butene (major product according to Zaitsev's rule) and 2-methyl-1-butene (minor product). Zaitsev's rule predicts the most substituted alkene will be favored.
4. E2 Reaction of 2-bromobutane with potassium tert-butoxide:
The bulky base preferentially abstracts a proton from the less hindered β-carbon, leading to the formation of 2-butene as the major product (Hofmann product). This is an exception to Zaitsev's rule due to steric hindrance.
5. Electrophilic Addition of HBr to propene:
The electrophilic addition follows Markovnikov's rule. The proton adds to the less substituted carbon, resulting in the formation of 2-bromopropane as the major product.
6. Nucleophilic Addition of Grignard Reagent to Aldehyde/Ketone:
A Grignard reagent (RMgX) acts as a nucleophile, adding to the carbonyl carbon of an aldehyde or ketone. The resulting alkoxide is then protonated to yield an alcohol. The regioselectivity is dictated by the carbonyl compound used.
Advanced Considerations: Beyond Simple Predictions
Predicting the major product often requires a more nuanced approach than simply identifying the dominant reaction mechanism. Several advanced concepts come into play:
-
Kinetic versus Thermodynamic Control: Sometimes, the major product is determined by the relative rates of formation (kinetic control) rather than the relative stabilities of the products (thermodynamic control). Temperature plays a key role in determining which control predominates.
-
Competing Reactions: In many instances, multiple reactions can occur simultaneously. Understanding the relative rates of these competing reactions is critical for accurate prediction of the major product.
-
Complex Reaction Pathways: Some reactions involve multiple steps and intermediates, making product prediction more challenging. Careful analysis of each step is crucial.
Conclusion: Mastering Product Prediction
Predicting the major product of an organic reaction is a multifaceted skill honed through practice and a solid understanding of reaction mechanisms, kinetics, and the influence of various factors. By systematically considering the mechanistic pathway, the influence of sterics, electronics, and reaction conditions, one can confidently determine the likely outcome. This article serves as a comprehensive resource, providing a solid foundation for tackling more complex reactions and developing a deeper appreciation for the intricate world of organic chemistry. Continued study and problem-solving are vital for refining your predictive abilities. Remember that practice is key to mastering this crucial aspect of organic chemistry. By diligently working through problems and analyzing different scenarios, you will significantly enhance your understanding and ability to predict major products with increasing accuracy.
Latest Posts
Latest Posts
-
Hans Selyes Definition Of Stress Is Considered
Mar 19, 2025
-
How Can Presenters Best Address Two Sides Of An Issue
Mar 19, 2025
-
The Ability To Make Things Move Or Change
Mar 19, 2025
-
2019 International Practice Exam Mcq Ap Lit
Mar 19, 2025
-
Harry Potter And The Deathly Hallows Ar Test Answers
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about What Is The Major Product Of The Following Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
