What Is The Relationship Between Antigens And Antibodies Quizlet
Breaking News Today
Mar 18, 2025 · 6 min read
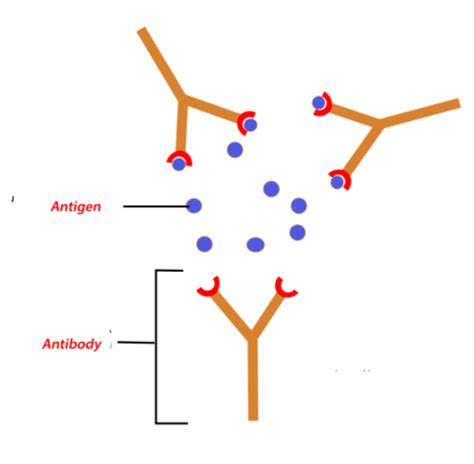
Table of Contents
What is the Relationship Between Antigens and Antibodies? A Comprehensive Guide
The relationship between antigens and antibodies is fundamental to the human immune system's ability to defend against infection and disease. Understanding this intricate dance is crucial to comprehending immunology and its implications for health and disease. This article will delve deep into the antigen-antibody interaction, exploring their definitions, structures, mechanisms of action, and clinical significance. We'll also address common misconceptions and explore relevant examples.
What are Antigens?
Antigens are substances that trigger an immune response. Essentially, they're molecules that the body recognizes as "foreign" or "non-self." This recognition leads to the production of antibodies, specialized proteins designed to neutralize or eliminate the antigen. Antigens can be various things including:
- Proteins: Many bacterial and viral surface proteins are potent antigens. These proteins, unique to the invading organism, act as molecular identifiers that alert the immune system to the presence of a pathogen.
- Polysaccharides: These complex carbohydrates, found on the surfaces of bacteria, fungi, and other microorganisms, also function as strong antigens. Their complex structures provide numerous epitopes, or sites for antibody binding.
- Lipids: While less common than proteins or polysaccharides, certain lipids can act as antigens, particularly when combined with other molecules.
- Nucleic acids: DNA and RNA, although typically located within the cell, can also act as antigens, especially when released from damaged cells or viruses.
Types of Antigens:
Antigens aren't a homogenous group. They can be categorized based on several factors:
- Exogenous antigens: These antigens originate from outside the body. They enter the body through various routes like inhalation, ingestion, or injection. Examples include bacterial toxins, pollen, and environmental allergens.
- Endogenous antigens: These antigens originate from within the body. They are typically produced by infected cells or cancerous cells. The immune system recognizes them as "self" gone rogue.
- Autoantigens: These are self-antigens that the immune system mistakenly identifies as foreign, triggering an autoimmune response. This self-recognition leads to the destruction of healthy tissues and organs.
- Alloantigens: These antigens are found on the surface of cells from different individuals of the same species. They are the basis for tissue rejection in organ transplantation and blood type incompatibilities.
What are Antibodies?
Antibodies, also known as immunoglobulins (Ig), are glycoproteins produced by plasma cells (differentiated B cells) in response to the presence of an antigen. They are Y-shaped molecules with specific binding sites designed to recognize and attach to a particular antigen. This recognition is remarkably precise; antibodies can differentiate between subtly different antigens.
Antibody Structure and Function:
The Y-shape of an antibody is crucial to its function. It consists of:
- Fab regions (Fragment antigen-binding): These are the "arms" of the Y, each containing a variable region (hypervariable regions) that binds specifically to a particular epitope on the antigen. The variability in this region allows antibodies to target a vast range of different antigens.
- Fc region (Fragment crystallizable): This is the "stem" of the Y. It interacts with other components of the immune system, such as complement proteins and phagocytic cells, triggering effector functions that eliminate the antigen.
Antibody Isotypes:
There are five major isotypes of antibodies (IgG, IgM, IgA, IgE, and IgD), each with distinct characteristics and functions. For example:
- IgG: The most abundant antibody in the blood, providing long-term immunity.
- IgM: The first antibody produced during an immune response.
- IgA: The primary antibody found in mucosal secretions (saliva, tears, mucus).
- IgE: Involved in allergic reactions and parasitic infections.
- IgD: Its function is less well understood, but it's found on the surface of B cells.
The Antigen-Antibody Interaction: A Detailed Look
The core of the immune response lies in the specific binding of an antibody to its corresponding antigen. This interaction, often likened to a "lock and key" mechanism, involves several steps:
- Antigen Recognition: The antibody's Fab region binds to a specific epitope on the antigen. The fit between the antibody and the epitope must be precise for binding to occur.
- Antigen-Antibody Complex Formation: The binding of the antibody to the antigen forms an antigen-antibody complex. This complex is crucial because it initiates a cascade of events leading to the antigen's neutralization or elimination.
- Effector Functions: The formation of the antigen-antibody complex triggers several effector functions, depending on the antibody isotype. These functions include:
- Neutralization: Antibodies block the antigen's ability to bind to cells or receptors, preventing infection or damage.
- Opsonization: Antibodies coat the antigen, making it more easily recognized and engulfed by phagocytic cells (macrophages and neutrophils).
- Complement Activation: Antibodies bind to complement proteins, triggering a cascade of reactions that lead to the lysis (destruction) of the antigen.
- Antibody-dependent cell-mediated cytotoxicity (ADCC): Antibodies bind to the antigen, marking it for destruction by natural killer (NK) cells.
Clinical Significance of the Antigen-Antibody Relationship
The antigen-antibody relationship is central to various diagnostic and therapeutic applications in medicine.
- Immunoassays: These laboratory tests detect the presence of either antigens or antibodies in a sample. Examples include ELISA (enzyme-linked immunosorbent assay) and Western blotting. These tests are used to diagnose infectious diseases, autoimmune disorders, and allergies.
- Immunotherapy: This type of therapy uses antibodies to target specific antigens associated with cancer cells or other disease-causing agents. Monoclonal antibodies are a prime example of this therapy.
- Vaccination: Vaccines introduce weakened or inactive forms of pathogens or their antigens into the body. This triggers an immune response, producing memory B cells and antibodies that provide long-term protection against future infections.
- Blood Typing: Blood type compatibility testing relies on the identification of antigens (agglutinogens) on red blood cells. Antibodies (agglutinins) in the plasma react with incompatible blood types, potentially causing dangerous agglutination (clumping).
Common Misconceptions about Antigens and Antibodies
Several misconceptions surround antigens and antibodies. Addressing these is crucial for a comprehensive understanding:
- Antigens always cause disease: While many antigens are associated with pathogens, many harmless antigens exist in our environment that don't necessarily cause disease.
- All antibodies are the same: The five major antibody isotypes (IgG, IgM, IgA, IgE, IgD) have different structures, functions, and locations in the body.
- Antigen-antibody binding is irreversible: While the binding is highly specific and strong, it's not permanently irreversible. The complex can dissociate under certain conditions.
Beyond the Basics: Advanced Concepts
The antigen-antibody interaction is a complex field with ongoing research. Here are some advanced concepts:
- Epitope Mapping: Determining the precise location and structure of the epitopes on an antigen is crucial for understanding its interaction with antibodies.
- Antibody Engineering: Scientists are developing new antibodies with improved properties for diagnostic and therapeutic applications, including improved binding affinity, enhanced effector functions, and reduced side effects.
- Immune System Regulation: The immune system tightly regulates the production of antibodies to prevent excessive inflammation and autoimmune responses. This intricate regulatory network involves various cells and signaling molecules.
- Immunological Memory: Following an infection or vaccination, the immune system retains memory B cells that quickly produce antibodies upon re-exposure to the same antigen. This is the basis of long-term immunity.
Conclusion:
The relationship between antigens and antibodies is central to the adaptive immune system’s ability to combat infection and maintain health. This intricate interaction involves specific recognition, complex binding, and diverse effector functions. Understanding this relationship is critical for diagnosing and treating diseases, developing vaccines, and advancing immunotherapeutic strategies. Further research continues to refine our understanding of this fundamental process, paving the way for innovative advancements in medicine and public health. This comprehensive guide provides a strong foundation for further exploration into the fascinating world of immunology.
Latest Posts
Latest Posts
-
Combustion Begins When A Fuel Is Heated To Its
May 09, 2025
-
Alcohol And Its Effects On The Body Worksheet Answers
May 09, 2025
-
The Four Principles That Guide Assistive Technology
May 09, 2025
-
The Team Will Play First Game On Saturday
May 09, 2025
-
Which Structure Is Indicated By The Arrow
May 09, 2025
Related Post
Thank you for visiting our website which covers about What Is The Relationship Between Antigens And Antibodies Quizlet . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.