What Is The Relationship Between The Following Compounds
Breaking News Today
Mar 24, 2025 · 6 min read
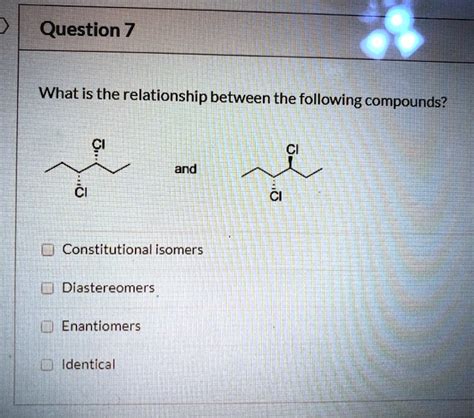
Table of Contents
Unveiling the Interconnected World of Chemical Compounds: Exploring Relationships Through Structure, Reactivity, and Synthesis
Understanding the relationships between different chemical compounds is fundamental to chemistry. This intricate web of connections stems from shared structural motifs, similar reactivity patterns, and intertwined synthetic pathways. This article delves into the multifaceted relationships between various chemical compounds, highlighting how seemingly disparate molecules can be linked through a deeper understanding of their chemical properties and behaviors. We will explore these relationships by examining several key aspects: structural similarities, functional group relationships, reactivity patterns, and synthetic connections.
I. Structural Similarities: The Foundation of Chemical Relationships
The most direct relationship between compounds often lies in their structural similarities. This can range from identical backbones with differing substituents to analogous frameworks with varied arrangements of atoms. Identifying these structural similarities is crucial for predicting and understanding their properties.
A. Isomerism: A Tale of Two (or More) Molecules
Isomers are molecules with the same molecular formula but different structural arrangements. This difference can manifest in various forms:
-
Constitutional Isomers: These isomers differ in the connectivity of their atoms. For example, butane and methylpropane are constitutional isomers, both having the formula C₄H₁₀ but differing in the arrangement of carbon atoms. Understanding constitutional isomerism is vital in understanding the diverse properties that can arise from even slight changes in atomic connectivity.
-
Stereoisomers: These isomers possess the same connectivity but differ in the spatial arrangement of their atoms. This category encompasses several sub-types:
-
Enantiomers: These are non-superimposable mirror images, also known as chiral molecules. A classic example is lactic acid, which exists in two enantiomeric forms with distinct biological activities. Understanding chirality is crucial in fields like pharmaceuticals, where one enantiomer might be therapeutically active while the other is inactive or even harmful.
-
Diastereomers: These stereoisomers are not mirror images of each other. They can differ in their physical and chemical properties, and examples include cis-trans isomers in alkenes and different anomers of carbohydrates.
-
B. Homologous Series: A Family Resemblance
Homologous series are groups of compounds that share a similar structural formula and differ by a repeating unit, typically a CH₂ group. Alkanes, alkenes, and alkynes are classic examples. The members of a homologous series exhibit a gradual change in properties as the chain length increases, providing a predictable pattern for understanding their behavior.
C. Functional Group Relationships: The Heart of Reactivity
Functional groups are specific groups of atoms within a molecule that are responsible for its characteristic chemical reactions. Compounds with similar functional groups often exhibit similar reactivity patterns. For instance, alcohols (containing the -OH group), carboxylic acids (-COOH), and amines (-NH₂) all undergo reactions characteristic of their respective functional groups, allowing for predictable chemical transformations.
II. Reactivity Patterns: Predicting Chemical Behavior
The reactivity of a compound is intrinsically linked to its structure, particularly its functional groups. Understanding these relationships allows us to predict how a compound will behave in different chemical environments.
A. Electrophilic and Nucleophilic Reactions: A Dance of Charges
Many organic reactions involve the interaction of electrophiles (electron-deficient species) and nucleophiles (electron-rich species). Functional groups determine the compound's ability to act as either an electrophile or a nucleophile, thereby governing the types of reactions it can undergo. For example, carbonyl compounds (containing the C=O group) act as electrophiles, while Grignard reagents act as nucleophiles, resulting in a variety of addition reactions.
B. Acid-Base Reactions: Proton Transfer and Equilibrium
Acid-base reactions are fundamental to chemistry, with the strength of an acid or base directly related to its structure. For example, carboxylic acids are stronger acids than alcohols due to the electron-withdrawing effect of the carbonyl group, stabilizing the conjugate base.
C. Oxidation-Reduction Reactions: Electron Transfer and Changes in Oxidation State
Oxidation-reduction reactions involve the transfer of electrons between compounds, resulting in a change in their oxidation states. For example, alcohols can be oxidized to aldehydes or ketones, and aldehydes can be further oxidized to carboxylic acids. The ease of oxidation depends on the structure of the alcohol and the oxidizing agent employed.
III. Synthetic Connections: Building Complex Molecules from Simpler Precursors
The synthesis of complex molecules often relies on understanding the relationships between simpler compounds and their reactivity. Many synthetic routes involve a series of transformations, each building upon the previous one to ultimately obtain the desired product.
A. Retrosynthetic Analysis: Working Backwards from the Target
Retrosynthetic analysis is a powerful tool for planning organic syntheses. This involves breaking down a complex target molecule into simpler precursors until readily available starting materials are reached. This approach helps strategize the synthesis by identifying key functional group transformations and reaction sequences.
B. Protecting Groups: Temporary Modifications to Enhance Selectivity
Protecting groups are used to temporarily mask certain functional groups during a synthesis, preventing unwanted side reactions. This strategy allows for selective transformations on other reactive sites within the molecule. Once the desired transformations are complete, the protecting groups are removed, restoring the original functionality.
C. Multi-step Syntheses: Building Complexity Through Sequential Reactions
The synthesis of many complex molecules requires a multi-step process, with each step building upon the previous one. Careful planning and optimization are crucial for achieving high yields and selectivity in such syntheses. This often involves choosing specific reagents and reaction conditions to favor the desired product over undesired byproducts.
IV. Advanced Relationships: Exploring Interdisciplinary Connections
The relationships between compounds extend beyond simple structural and reactivity considerations. Understanding these broader connections is crucial for advancing chemical knowledge and its applications.
A. Structure-Activity Relationships (SAR): Linking Structure to Biological Activity
SAR studies investigate the relationship between the chemical structure of a molecule and its biological activity. This is particularly important in drug discovery, where subtle changes in structure can significantly alter a compound's efficacy, toxicity, and other pharmacokinetic properties.
B. Cheminformatics and Molecular Modeling: Predicting Properties and Designing Molecules
Cheminformatics and molecular modeling utilize computational techniques to predict the properties and reactivity of molecules, often without the need for extensive laboratory experiments. These methods are invaluable for identifying promising drug candidates and designing new materials with specific properties.
C. Green Chemistry Principles: Sustainable Synthesis and Environmental Considerations
Green chemistry emphasizes developing environmentally friendly synthetic methods that minimize waste and use renewable resources. This approach requires a deep understanding of the relationships between different compounds and their potential environmental impacts, favoring less harmful reagents and processes.
V. Conclusion: A Dynamic and Interconnected World
The relationships between chemical compounds are complex and multifaceted, spanning structural similarities, reactivity patterns, and synthetic pathways. Understanding these connections is fundamental to advancing chemical knowledge, predicting chemical behavior, and designing new molecules with desired properties. From the simple relationship between isomers to the sophisticated interplay between structure and biological activity, exploring these connections allows us to unlock a deeper understanding of the chemical world around us. Continued exploration and refinement of these relationships will continue to drive innovation in various fields, including pharmaceuticals, materials science, and environmental sustainability. As our understanding of chemical compounds grows, so too will our ability to harness their potential for the betterment of society.
Latest Posts
Latest Posts
-
A Diamond Tip Microdermabrasion Device Is Also Known As
Mar 29, 2025
-
Which Vitamin Does Not Have Antioxidant Properties
Mar 29, 2025
-
The Last Line Of A Proof Represents
Mar 29, 2025
-
Many At The Continental Congress Were Skeptical
Mar 29, 2025
-
When Relaying Patient Information Via Radio Communications Should Be
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about What Is The Relationship Between The Following Compounds . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
