What Is The Vertical Column In The Periodic Table Called
Breaking News Today
Mar 26, 2025 · 5 min read
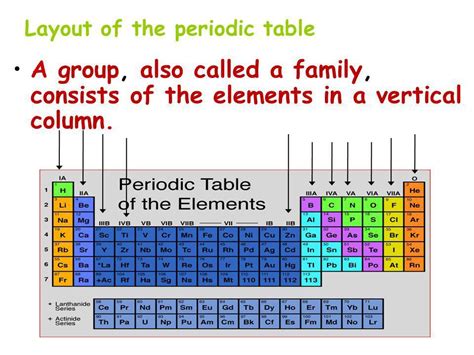
Table of Contents
What is the Vertical Column in the Periodic Table Called? A Deep Dive into Groups and Families
The periodic table, that iconic grid of elements, is more than just a neatly organized list. It's a powerful tool that reveals fundamental relationships between atoms and their properties. Understanding its structure is key to comprehending chemistry. A crucial aspect of this structure is the vertical column, which is officially known as a group or sometimes referred to as a family. This article will explore the significance of these vertical columns, delving into their characteristics, trends, and the underlying reasons for their similarities.
Understanding Groups: More Than Just a Column
The vertical columns, or groups, of the periodic table are not just arbitrary arrangements; they represent elements with strikingly similar chemical properties. This similarity stems from the number of electrons in their outermost shell, called the valence shell. Elements within the same group have the same number of valence electrons, leading to predictable patterns in their reactivity and bonding behavior. This shared characteristic is the foundation for their classification into families.
Valence Electrons: The Key to Group Similarity
The valence electrons are the key players determining an element's position in a group and dictating its chemical behavior. These are the electrons involved in chemical bonding, determining how an element will interact with other elements to form compounds. Elements in the same group possess the same number of valence electrons, leading to similar chemical reactions and compound formations. For example, all elements in Group 1 (alkali metals) have one valence electron, contributing to their high reactivity and tendency to form +1 ions.
Predicting Properties Based on Group Membership
The beauty of the periodic table lies in its predictive power. Once you understand the properties of one element in a group, you can often make reasonably accurate predictions about the properties of other elements in the same group. This predictive capability extends to various properties including:
- Reactivity: Group 1 elements are highly reactive, while Group 18 (noble gases) are largely inert. This drastic difference is directly related to their valence electron configuration.
- Oxidation States: The tendency of an element to lose or gain electrons to achieve a stable electron configuration is reflected in its oxidation state. Elements within a group generally exhibit similar oxidation states.
- Ionization Energy: The energy required to remove an electron from an atom is known as ionization energy. This value shows predictable trends within a group.
- Electronegativity: This property measures an atom's ability to attract electrons towards itself in a chemical bond. Electronegativity trends are also apparent within groups.
- Atomic Radius: The size of an atom tends to increase as you move down a group due to the addition of electron shells.
Exploring Key Groups and Their Characteristics
Let's delve into some specific groups, highlighting their unique properties and the reasons behind their characteristics:
Group 1: Alkali Metals
The alkali metals (Lithium, Sodium, Potassium, Rubidium, Caesium, and Francium) are characterized by their extreme reactivity. This high reactivity is a direct consequence of their single valence electron, which they readily lose to form +1 ions. They are soft, silvery-white metals that react vigorously with water, producing hydrogen gas and a strongly alkaline solution.
Group 2: Alkaline Earth Metals
Alkaline earth metals (Beryllium, Magnesium, Calcium, Strontium, Barium, and Radium) have two valence electrons, making them less reactive than alkali metals but still quite reactive compared to other groups. They also readily form +2 ions. These metals are harder and denser than alkali metals and have various applications, ranging from construction materials (calcium) to lightweight alloys (magnesium).
Group 17: Halogens
Halogens (Fluorine, Chlorine, Bromine, Iodine, and Astatine) are highly reactive non-metals. They have seven valence electrons, meaning they only need one more electron to achieve a stable octet. This strong tendency to gain an electron leads to their formation of -1 ions. Halogens exhibit a range of physical states, with fluorine and chlorine being gases, bromine a liquid, and iodine a solid.
Group 18: Noble Gases
Noble gases (Helium, Neon, Argon, Krypton, Xenon, and Radon) are unique for their extreme inertness. They have a full valence shell (eight electrons, except for helium with two), making them exceptionally stable and unreactive. Their lack of reactivity has historically limited their use, but recent advancements have led to some applications in specialized areas like lighting and lasers.
The Importance of Group Trends in Chemistry
Understanding the trends within groups is essential for predicting chemical reactions and properties. This knowledge forms the backbone of various chemical concepts:
- Predicting Reaction Products: Knowing the number of valence electrons allows chemists to predict the likely products of chemical reactions.
- Designing New Materials: The properties of elements within a group can be tailored to create materials with specific characteristics.
- Developing New Technologies: Understanding group trends has led to the development of various technologies, including new batteries, catalysts, and medical treatments.
Beyond the Main Groups: Transition Metals and Other Elements
The periodic table also includes transition metals and other elements which don't perfectly fit the strict valence electron rules of the main groups. Transition metals, located in the middle of the table, have variable oxidation states and often exhibit colorful compounds due to the involvement of d-electrons in bonding. Lanthanides and actinides, at the bottom of the table, form a separate block with unique electronic configurations and properties.
Conclusion: The Vertical Column is a Key to Understanding Chemistry
The vertical columns, or groups, of the periodic table are far more than simple organizational features. They are fundamental to understanding the chemical behavior and properties of elements. The shared number of valence electrons in each group leads to predictable trends in reactivity, oxidation states, and other properties, enabling chemists to predict reactions, design materials, and develop new technologies. Mastering the concepts of groups and families is crucial for anyone seeking a deeper understanding of chemistry and its applications in the world around us. Further exploration into individual groups and their specific characteristics can lead to a much more comprehensive and nuanced understanding of the periodic table's power and predictive ability. The more you explore, the more you'll appreciate the elegance and utility of this essential chemical tool.
Latest Posts
Latest Posts
-
Which Of These Is The Best Example Of Fragmentation
Mar 27, 2025
-
The Most Likely Cause Of Bedding In This Image Is
Mar 27, 2025
-
Match Each Definition With The Correct Term
Mar 27, 2025
-
Time Values In Music Are Expressed In Absolute Terms
Mar 27, 2025
-
Which Of The Following Does Not Relate To System Design
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about What Is The Vertical Column In The Periodic Table Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
