Which Argument Best Explains The Charge Of An Atomic Nucleus
Breaking News Today
Mar 12, 2025 · 5 min read
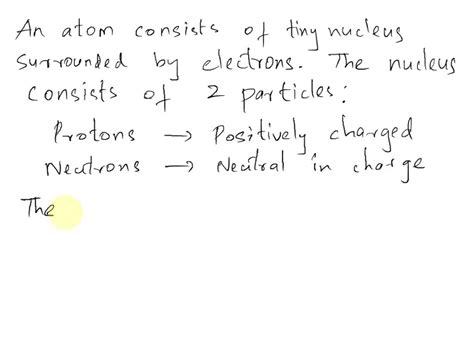
Table of Contents
Which Argument Best Explains the Charge of an Atomic Nucleus?
The charge of an atomic nucleus, a fundamental concept in physics and chemistry, is a cornerstone of our understanding of matter. While seemingly straightforward – positively charged – the why behind this positive charge is a journey through the subatomic world, necessitating a deep dive into several competing arguments and models. This article will examine the prevailing argument and compare it to alternative explanations, ultimately concluding which best supports the observed phenomena.
The Standard Model and the Proton's Charge
The most accepted explanation for the positive charge of an atomic nucleus rests firmly on the Standard Model of particle physics. This model posits that the nucleus is composed primarily of two types of particles: protons and neutrons. Crucially, protons possess a positive elementary charge (+1e), while neutrons are electrically neutral. The overall positive charge of the nucleus is therefore a direct consequence of the number of protons it contains – the atomic number (Z).
The Quark Composition of Protons: A Deeper Dive
To understand the proton's positive charge even further, we need to consider its constituent particles: quarks. Protons are made up of three quarks: two up quarks and one down quark. Up quarks carry a charge of +2/3e, and down quarks carry a charge of -1/3e. Therefore, the net charge of a proton is (2/3e) + (2/3e) + (-1/3e) = +1e. This fundamental level explanation provides a concrete basis for the proton's, and consequently the nucleus', positive charge.
Electromagnetic Interactions: The Glue that Binds (and Repels)
The positive charges of the protons within the nucleus create a strong electromagnetic repulsion. This repulsion would naturally cause the nucleus to fly apart, were it not for the incredibly powerful strong nuclear force. This force, much stronger than the electromagnetic force at short distances, overcomes the electrostatic repulsion, binding the protons (and neutrons) together to form a stable nucleus. The balance between these two fundamental forces is paramount to the existence of stable atoms and the structure of matter as we know it.
Alternative Arguments and Their Limitations
While the Standard Model offers a comprehensive and largely accepted explanation, several alternative arguments have been proposed throughout the history of physics. However, these often lack the empirical evidence and predictive power of the Standard Model.
Early Atomic Models and Their Shortcomings
Early models of the atom, like the Thomson "plum pudding" model, proposed a diffuse positive charge throughout the atom, with electrons embedded within it. This model was quickly superseded by Rutherford's gold foil experiment, which definitively demonstrated the existence of a concentrated, positively charged nucleus. These early models failed to account for the quantized nature of charge and the existence of fundamental particles like protons and quarks.
Hypothetical Charges: Unfalsifiable Speculation
Some speculative arguments propose the existence of hitherto undiscovered particles or forces that could contribute to the nucleus's charge in a different way. However, such arguments are difficult to test and often lack any experimental backing. Without verifiable evidence, these hypothetical explanations remain unfalsifiable and thus lack the scientific rigor of the Standard Model.
The Role of Neutrons: A Clarification
While neutrons are electrically neutral, their presence within the nucleus is crucial for nuclear stability. Neutrons help to mediate the strong nuclear force, effectively buffering the repulsive forces between protons. However, they do not contribute directly to the overall positive charge of the nucleus. Their role is essential for maintaining nuclear integrity but not for explaining the positive charge itself.
Experimental Evidence Supporting the Standard Model
Numerous experiments provide robust support for the Standard Model's explanation of the nucleus's positive charge:
- Rutherford's Gold Foil Experiment: This landmark experiment directly demonstrated the existence of a small, dense, positively charged nucleus within the atom.
- Particle Accelerator Experiments: High-energy particle collisions have consistently confirmed the existence and properties of protons and quarks, supporting the Standard Model's description of their charges.
- Spectroscopic Analysis: The spectral lines emitted by atoms provide strong evidence for the quantized nature of charge and the specific energy levels associated with electrons interacting with the positively charged nucleus.
- Nuclear Reactions: Nuclear reactions, such as alpha decay and fission, provide further experimental validation of the nuclear structure and the role of protons in determining the charge.
Addressing Potential Objections and Misconceptions
Some common misconceptions regarding the nuclear charge need clarification:
- The Nucleus as a Simple Sum of Proton Charges: While the total positive charge is the sum of individual proton charges, the complexities of the strong nuclear force and quark interactions need to be acknowledged. It's not merely a simple addition.
- The Role of Dark Matter: Dark matter, a mysterious substance that makes up a large portion of the universe's mass, is not involved in the basic electrostatics of the atomic nucleus.
- Quantum Fluctuations and Charge Variations: While quantum fluctuations can lead to temporary charge imbalances, they do not alter the fundamental positive charge of the atomic nucleus over time.
Conclusion: The Standard Model's Triumph
In conclusion, the argument based on the Standard Model of particle physics provides the most compelling and experimentally verified explanation for the positive charge of the atomic nucleus. The Standard Model, incorporating the positive charge of the proton, its quark composition, and the mediating role of the strong nuclear force, offers a coherent and comprehensive picture that aligns with a vast body of experimental data. Alternative explanations lack the same level of empirical support and predictive power. The Standard Model’s robust framework continues to be the cornerstone of our understanding of matter at the subatomic level, leaving the explanation of the positive charge of the atomic nucleus firmly grounded in its established principles. While refinements and extensions to the model might be made in the future, its core tenets regarding nuclear charge remain remarkably consistent and well-supported by decades of scientific investigation.
Latest Posts
Latest Posts
-
Quotes From Act 5 Scene 1 Macbeth
Mar 12, 2025
-
Explain How Oil Paint Is Made What Is The Vehicle
Mar 12, 2025
-
Which Is An Example Of A Historical Challenge Of Stamis
Mar 12, 2025
-
You Are Marking An Audio Recording Of A Conversation
Mar 12, 2025
-
Companies Attempted To Intimidate Union Organizers By
Mar 12, 2025
Related Post
Thank you for visiting our website which covers about Which Argument Best Explains The Charge Of An Atomic Nucleus . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
