Which Diagram Best Represents A Polar Molecule
Breaking News Today
Apr 01, 2025 · 6 min read
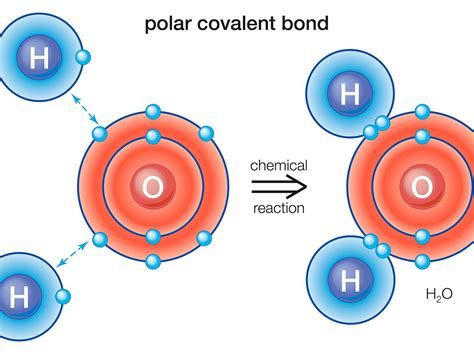
Table of Contents
Which Diagram Best Represents a Polar Molecule? Understanding Molecular Geometry and Dipole Moments
Understanding molecular polarity is crucial in chemistry, as it dictates many physical and chemical properties of substances. A polar molecule possesses a net dipole moment, meaning it has a slightly positive end and a slightly negative end due to an uneven distribution of electron density. But how do we visually represent this, and which diagram best captures the essence of a polar molecule? This article dives deep into the various diagrams used to represent molecules, focusing on which ones most effectively illustrate polarity. We'll explore the concepts of electronegativity, molecular geometry, and dipole moments to solidify our understanding.
What Makes a Molecule Polar?
Before we dive into diagrams, let's establish the fundamental principles behind molecular polarity. The key factors are:
- Electronegativity: This refers to an atom's ability to attract electrons within a chemical bond. Elements with higher electronegativity (like oxygen, nitrogen, and fluorine) pull electrons more strongly towards themselves.
- Bond Polarity: When two atoms with different electronegativities bond, the electrons are unequally shared, creating a polar bond. The more electronegative atom develops a partial negative charge (δ-), while the less electronegative atom develops a partial positive charge (δ+).
- Molecular Geometry: The three-dimensional arrangement of atoms in a molecule is crucial. Even if a molecule contains polar bonds, the molecule itself might be nonpolar if the bond dipoles cancel each other out due to symmetry.
In essence, a polar molecule arises from a combination of polar bonds and an asymmetrical molecular geometry that prevents the bond dipoles from canceling each other.
Diagrams Representing Molecular Structure and Polarity
Several diagrams can be used to represent molecules, each with strengths and weaknesses in illustrating polarity:
1. Lewis Structures
Lewis structures show the bonding and lone pairs of electrons around each atom. While useful for understanding bonding, they are not ideal for depicting polarity directly. They illustrate bond polarity through the difference in electronegativity between bonded atoms, but don't visually represent the overall dipole moment of the molecule. For example, a Lewis structure of water (H₂O) shows the polar O-H bonds, but doesn't explicitly show the overall bent shape and resulting dipole moment.
Example: The Lewis structure of water shows the oxygen atom sharing electrons with two hydrogen atoms, but it does not illustrate the bent molecular geometry.
2. 3D Molecular Models (Ball-and-Stick and Space-Filling)
3D models provide a much better visual representation of molecular geometry.
-
Ball-and-stick models: These models represent atoms as spheres and bonds as sticks. They effectively show bond angles and the spatial arrangement of atoms, making it easier to visualize if bond dipoles cancel out or result in a net dipole moment.
-
Space-filling models: These models represent atoms as spheres with sizes proportional to their atomic radii. They provide a more realistic representation of the molecule's shape and electron density, enhancing the understanding of electron distribution and thus, polarity.
Both ball-and-stick and space-filling models are excellent for visualizing molecular geometry and, consequently, polarity. However, they don't explicitly indicate the direction or magnitude of the dipole moment.
3. Vector Diagrams
Vector diagrams are the most effective method for explicitly showing the molecule's dipole moment. Each polar bond is represented by a vector, an arrow pointing from the positive (δ+) end to the negative (δ-) end. The length of the vector represents the magnitude of the bond dipole, reflecting the difference in electronegativity. The resultant dipole moment of the molecule is the vector sum of all individual bond dipoles. If the vectors cancel each other out, the molecule is nonpolar; if there's a net resultant vector, the molecule is polar.
Example: In a water molecule, two vectors point from the hydrogen atoms towards the oxygen atom. Because of the bent geometry, these vectors do not cancel each other, resulting in a net dipole moment pointing towards the oxygen atom. This clearly demonstrates water's polarity.
4. Dipole Moment Arrows
A single arrow is often used to represent the overall molecular dipole moment. This arrow points from the partial positive end of the molecule to the partial negative end. The length of the arrow (although not always to scale in simplified diagrams) generally represents the magnitude of the dipole moment. This is a simple, yet effective, way to summarize the molecule's polarity. This is often used in conjunction with other diagrams like 3D models.
Comparing the Diagrams: Which is Best?
While Lewis structures are essential for understanding bonding, they are insufficient for directly showing polarity. 3D models offer excellent visualizations of molecular geometry, providing context for understanding dipole moment cancellation. However, vector diagrams provide the most direct and unambiguous representation of a molecule's polarity. They graphically demonstrate how individual bond dipoles combine to form the overall molecular dipole moment.
Therefore, a combination of a 3D model (ball-and-stick or space-filling) and a vector diagram is arguably the most effective way to represent a polar molecule. The 3D model provides the spatial context, showing the molecular geometry that leads to the overall dipole moment, while the vector diagram explicitly demonstrates the resultant dipole moment. The inclusion of a dipole moment arrow further simplifies the overall representation.
Examples: Illustrating Polarity with Different Diagrams
Let's consider a few examples to illustrate the differences in how each diagram represents polarity:
1. Carbon Dioxide (CO₂):
- Lewis Structure: Shows two double bonds between carbon and oxygen atoms.
- 3D Model (Linear): Shows a linear arrangement of atoms with the carbon atom in the center.
- Vector Diagram: Shows two bond dipoles pointing from the carbon atom towards the oxygen atoms. Because the molecule is linear, these dipoles cancel each other out, resulting in a zero net dipole moment. Therefore, CO₂ is nonpolar.
2. Water (H₂O):
- Lewis Structure: Shows two single bonds between oxygen and hydrogen atoms, with two lone pairs of electrons on the oxygen atom.
- 3D Model (Bent): Shows a bent molecular geometry with a bond angle of approximately 104.5 degrees.
- Vector Diagram: Shows two bond dipoles pointing from the hydrogen atoms towards the oxygen atom. Due to the bent geometry, these dipoles do not cancel each other out, resulting in a net dipole moment. Therefore, H₂O is polar.
3. Ammonia (NH₃):
- Lewis Structure: Shows three single bonds between nitrogen and hydrogen atoms, with one lone pair of electrons on the nitrogen atom.
- 3D Model (Trigonal Pyramidal): Shows a trigonal pyramidal geometry with the nitrogen atom at the apex.
- Vector Diagram: Shows three bond dipoles pointing from the hydrogen atoms towards the nitrogen atom. The lone pair contributes to the overall asymmetry, preventing the dipoles from canceling out. Therefore, NH₃ is polar.
Conclusion: Visualizing Polarity for a Clearer Understanding
Understanding molecular polarity is fundamental in chemistry. While various diagrams help visualize molecular structure and bonding, a combination of 3D models and vector diagrams offers the most comprehensive and clear representation of a molecule's polarity. This combination allows for a thorough understanding of how molecular geometry and bond polarity interplay to determine the overall dipole moment of a molecule, leading to a better grasp of its physical and chemical properties. Remember, a clear visual representation of this concept is key to solidifying your understanding of chemical behavior. Utilizing multiple visualization techniques allows for a deeper comprehension of the intricate world of molecular polarity.
Latest Posts
Latest Posts
-
What Is A Triangle Shaped Deposit Of Sediment
Apr 02, 2025
-
A Type Of Verbal Behavior With The Response
Apr 02, 2025
-
With A Good Mask To Face Seal
Apr 02, 2025
-
The Olfactory Bulbs Of The Sheep
Apr 02, 2025
-
A Police Officer Is Using A Radar Device
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Which Diagram Best Represents A Polar Molecule . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
