Which Of The Following Are Properties Of Hydrocarbons
Breaking News Today
Mar 12, 2025 · 6 min read
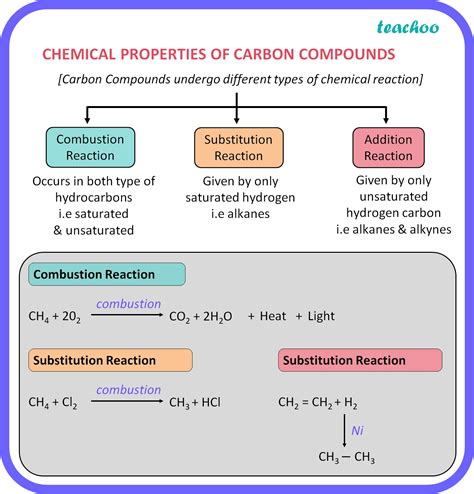
Table of Contents
Which of the Following are Properties of Hydrocarbons? A Comprehensive Guide
Hydrocarbons, the fundamental building blocks of organic chemistry, are organic compounds consisting solely of hydrogen and carbon atoms. Their diverse structures lead to a wide range of properties, making them crucial in various industries, from fuels to plastics. This comprehensive guide will delve into the key properties of hydrocarbons, exploring their physical and chemical characteristics, and addressing common misconceptions.
Physical Properties of Hydrocarbons
The physical properties of hydrocarbons are largely determined by two key factors: molecular weight and structure.
1. Molecular Weight and Intermolecular Forces
As the molecular weight of a hydrocarbon increases, several physical properties change:
-
Boiling Point: Hydrocarbons exhibit relatively low boiling points compared to other organic compounds with similar molecular weights. However, boiling point increases with increasing molecular weight. This is because larger molecules experience stronger London dispersion forces (a type of van der Waals force), requiring more energy to overcome these intermolecular attractions and transition to the gaseous phase. Shorter chains have weaker intermolecular forces and therefore lower boiling points.
-
Melting Point: Similar to boiling point, the melting point also increases with increasing molecular weight. The stronger intermolecular forces in larger molecules make it more difficult to disrupt the organized crystalline structure in the solid phase. However, the melting point is also significantly influenced by the molecular structure, as will be discussed later.
-
Density: Hydrocarbons are generally less dense than water. Their density increases with increasing molecular weight, but they remain less dense than water, which is why oil floats on water.
2. Structure and its Influence
The structure of a hydrocarbon molecule—whether it's linear, branched, or cyclic—significantly impacts its physical properties:
-
Branching: Branched-chain hydrocarbons generally have lower boiling points and melting points than their straight-chain isomers. This is because branching reduces the surface area available for intermolecular interactions, weakening the London dispersion forces.
-
Isomerism: Isomers are molecules with the same molecular formula but different structural arrangements. Different isomers of the same hydrocarbon can have significantly different physical properties due to variations in their intermolecular forces.
-
Cyclic Structures: Cyclic hydrocarbons (those containing rings of carbon atoms) often have higher boiling points and melting points than their straight-chain counterparts of the same molecular weight. The ring structure enhances the surface area for intermolecular interactions.
-
Aromaticity: Aromatic hydrocarbons, characterized by a benzene ring structure, exhibit unique properties. They generally have higher boiling points and melting points than comparable aliphatic (non-aromatic) hydrocarbons. Aromaticity imparts significant stability to the molecule.
3. State of Matter at Room Temperature
The state of matter of a hydrocarbon at room temperature depends largely on its molecular weight:
-
Gases: Smaller hydrocarbons (e.g., methane, ethane, propane) are gases at room temperature due to their weak intermolecular forces.
-
Liquids: Medium-sized hydrocarbons (e.g., butane, octane) are liquids at room temperature.
-
Solids: Larger hydrocarbons (e.g., paraffin wax) are solids at room temperature.
Chemical Properties of Hydrocarbons
The chemical properties of hydrocarbons are primarily determined by the presence of relatively nonpolar C-H and C-C bonds. This results in their relatively low reactivity compared to many other organic compounds. However, they do undergo specific types of reactions.
1. Combustion
The most significant chemical reaction of hydrocarbons is combustion – reaction with oxygen. This is an exothermic reaction, releasing a large amount of heat and producing carbon dioxide and water as products. The complete combustion of hydrocarbons is represented by the general equation:
CxHy + (x + y/4)O2 → xCO2 + (y/2)H2O + Heat
Incomplete combustion, often occurring under conditions of limited oxygen supply, produces carbon monoxide (CO) and/or soot (carbon particles) in addition to carbon dioxide and water.
2. Substitution Reactions
Alkanes, saturated hydrocarbons with only single bonds, undergo substitution reactions where a hydrogen atom is replaced by another atom or group. Halogenation (reaction with halogens like chlorine or bromine) is a common example:
CH4 + Cl2 → CH3Cl + HCl
This reaction requires ultraviolet (UV) light to initiate the process.
3. Addition Reactions
Alkenes (hydrocarbons with at least one carbon-carbon double bond) and alkynes (hydrocarbons with at least one carbon-carbon triple bond) readily undergo addition reactions. In these reactions, atoms or groups add across the multiple bond, breaking the pi bonds and forming new sigma bonds. Examples include:
-
Hydrogenation: Addition of hydrogen (H2) across the double or triple bond, converting an alkene or alkyne to an alkane. This reaction often requires a metal catalyst (like platinum or palladium).
-
Halogenation: Addition of halogens (Cl2, Br2) across the double or triple bond.
-
Hydration: Addition of water (H2O) across the double bond, forming an alcohol.
4. Oxidation Reactions
Hydrocarbons can undergo oxidation reactions, often resulting in the breaking of C-C or C-H bonds. Complete combustion is a form of oxidation. Other oxidation reactions can produce carboxylic acids, ketones, or aldehydes, depending on the structure of the hydrocarbon and the oxidizing agent used.
5. Cracking and Reforming
These processes are crucial in the petroleum industry for modifying the properties of hydrocarbons.
-
Cracking: Large hydrocarbon molecules are broken down into smaller, more useful molecules. This is often done using heat and/or a catalyst.
-
Reforming: The structure of hydrocarbons is altered, often to increase the octane rating of gasoline. This process involves isomerization, cyclization, and dehydrogenation reactions.
Identifying Hydrocarbons: Key Distinguishing Features
Several characteristics can help distinguish different types of hydrocarbons:
-
Saturated vs. Unsaturated: Saturated hydrocarbons (alkanes) contain only single bonds, while unsaturated hydrocarbons (alkenes and alkynes) contain double or triple bonds, respectively. This difference significantly impacts their reactivity.
-
Aliphatic vs. Aromatic: Aliphatic hydrocarbons are non-aromatic, while aromatic hydrocarbons contain at least one benzene ring. Aromatic compounds exhibit unique stability due to resonance.
-
Linear, Branched, or Cyclic: The arrangement of carbon atoms in the molecule affects its properties, as previously discussed.
-
Functional Groups: While hydrocarbons themselves lack functional groups (atoms or groups of atoms with characteristic chemical properties), the addition of functional groups to hydrocarbons creates a vast array of organic compounds with diverse properties.
Applications of Hydrocarbons
The wide range of properties exhibited by hydrocarbons translates into a vast array of applications:
-
Fuels: Hydrocarbons are the primary source of energy in the world, used as fuels for transportation (gasoline, diesel), heating (natural gas, propane), and electricity generation.
-
Plastics and Polymers: Many plastics and polymers are derived from hydrocarbons, providing materials for countless applications, from packaging to construction.
-
Solvents: Certain hydrocarbons are used as solvents in various industrial processes.
-
Lubricants: Hydrocarbons are used as lubricants in engines and machinery.
-
Waxes and Paraffins: These materials, derived from hydrocarbons, are used in candles, cosmetics, and other products.
Conclusion
Understanding the properties of hydrocarbons – both physical and chemical – is essential in numerous fields. From their role as primary energy sources to their use as building blocks for countless materials, their importance in modern society is undeniable. This exploration provides a foundation for further study into the fascinating world of organic chemistry and its applications. Remember to always consider safety precautions when working with hydrocarbons, especially due to their flammability.
Latest Posts
Latest Posts
-
All Of The Following Are Disadvantages Of A Corporation Except
May 09, 2025
-
Which Word Part Contains The Fundamental Meaning Of The Word
May 09, 2025
-
Tendons And Ligaments Are Composed Primarily Of
May 09, 2025
-
The Loss Of Information Through Nonuse Is Called
May 09, 2025
-
In Which Situation Should You Use High Beams
May 09, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Are Properties Of Hydrocarbons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.