Which Of The Following Best Explains Diffusion
Breaking News Today
Mar 30, 2025 · 6 min read
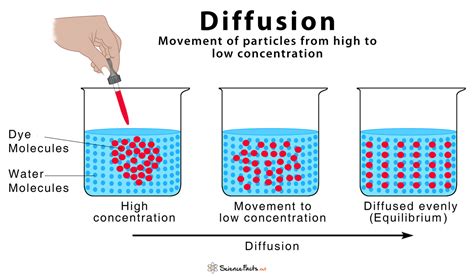
Table of Contents
Which of the Following Best Explains Diffusion? A Deep Dive into the Process
Diffusion, a ubiquitous process in nature and technology, is often simplified in textbooks. But understanding diffusion truly involves grasping its underlying mechanisms, variations, and impact across diverse fields. This article delves deep into the intricacies of diffusion, exploring various perspectives and clarifying common misconceptions. We'll examine several potential explanations and determine which best encapsulates the essence of diffusion.
Understanding the Basics: What is Diffusion?
Before exploring different explanations, let's establish a clear definition. Diffusion is the net movement of anything (for example, atom, ions, molecules, energy) from a region of higher concentration to a region of lower concentration. This movement continues until the substance is uniformly distributed throughout the space. This process is driven by the inherent tendency of systems to move towards a state of maximum entropy or disorder. Think of a drop of dye spreading in a glass of water – that's diffusion in action.
It's crucial to differentiate diffusion from other transport processes:
- Active transport: This requires energy input to move substances against a concentration gradient (from low to high concentration). Diffusion, in contrast, is a passive process.
- Bulk flow: This involves the movement of a large group of molecules due to pressure differences, as seen in blood flow through veins and arteries. Diffusion is a much more individualistic process at the molecular level.
Explanations of Diffusion: A Comparative Analysis
Several explanations attempt to capture the essence of diffusion. Let's examine some of them:
1. Random Molecular Motion: This is arguably the most fundamental explanation. Diffusion is a direct consequence of the constant, random motion of particles. Particles are in perpetual movement, colliding with each other and with the surrounding environment. This random movement, though seemingly chaotic, leads to a net movement from areas of high concentration to areas of low concentration. Over time, this random walk results in a uniform distribution.
Why this is a strong explanation: This explanation connects directly to the kinetic theory of matter, a well-established scientific principle. It avoids oversimplification and accounts for the probabilistic nature of diffusion.
Limitations: This explanation might be less intuitive for those unfamiliar with kinetic theory. It doesn't explicitly address the factors influencing the rate of diffusion.
2. Concentration Gradient as the Driving Force: This explanation focuses on the difference in concentration between two regions as the driving force behind diffusion. The steeper the concentration gradient (the larger the difference in concentration), the faster the rate of diffusion. Particles naturally move "down" the concentration gradient, seeking to equalize the concentrations.
Why this is a helpful explanation: It provides a practical, readily understandable description of diffusion. It directly links the rate of diffusion to a measurable quantity – the concentration gradient.
Limitations: This explanation alone doesn't fully explain why particles move down the concentration gradient. It's a description of the outcome, not the underlying mechanism.
3. Entropy Maximization: From a thermodynamic perspective, diffusion is a process that increases entropy, the measure of disorder or randomness in a system. A system with unevenly distributed particles has lower entropy than a system with uniformly distributed particles. Diffusion, by spreading particles, increases entropy, thus moving the system towards a state of thermodynamic equilibrium.
Why this is a powerful explanation: This explanation provides a deeper, more fundamental understanding of diffusion's driving force. It connects diffusion to the second law of thermodynamics, one of the most fundamental principles of physics.
Limitations: This explanation can be more abstract and challenging to grasp for those unfamiliar with thermodynamics. It may not be as immediately intuitive as the concentration gradient explanation.
4. A Combination of Explanations: The Most Accurate Perspective
The most comprehensive and accurate explanation of diffusion isn't a single statement but rather a combination of the above. The random motion of particles (kinetic theory) leads to a net movement down the concentration gradient, ultimately maximizing entropy. These three aspects are interconnected and inseparable. The concentration gradient acts as a convenient measure of the driving force, but the underlying cause is the inherent tendency of particles to move randomly and thus increase disorder.
Factors Affecting Diffusion Rate
The rate at which diffusion occurs isn't constant. Several factors influence this rate:
- Temperature: Higher temperatures increase particle kinetic energy, leading to faster diffusion.
- Concentration gradient: A steeper gradient results in faster diffusion.
- Mass of particles: Heavier particles diffuse more slowly than lighter ones.
- Distance: Diffusion rate decreases with increasing distance. The further particles need to travel, the longer it takes.
- Medium: The medium through which diffusion occurs greatly affects the rate. Diffusion is much faster in gases than in liquids, and even slower in solids. The viscosity and porosity of the medium play a significant role.
Diffusion in Different Contexts
Diffusion is a fundamental process with far-reaching implications across numerous fields:
- Biology: Diffusion is essential for nutrient transport in cells, gas exchange in lungs, and signal transduction in the nervous system. The movement of oxygen and carbon dioxide in our bodies relies heavily on diffusion.
- Chemistry: Diffusion plays a crucial role in chemical reactions, particularly in solutions. The rate of reactions often depends on how quickly reactants can diffuse together.
- Material science: Diffusion is vital in various material processing techniques, such as doping semiconductors and creating alloys. Controlled diffusion is used to enhance the properties of materials.
- Environmental science: Diffusion is significant in understanding pollutant dispersion in air and water. Understanding diffusion patterns helps in modeling and mitigating environmental contamination.
- Physics: Diffusion is studied in various physical systems, including gases, liquids, and solids. It is a crucial concept in statistical mechanics and thermodynamics.
Common Misconceptions about Diffusion
Several misconceptions frequently surround diffusion:
- Diffusion is always fast: While diffusion can be rapid in certain contexts, it's often a relatively slow process, especially over long distances.
- Diffusion only applies to molecules: Diffusion applies to any entity that undergoes random motion, including heat, energy, and even information.
- Diffusion requires a specific mechanism: Diffusion is a passive process; it doesn't require any specific external mechanism or force other than random thermal motion.
Conclusion: A Holistic Understanding of Diffusion
In conclusion, the most accurate explanation of diffusion is a multifaceted one, incorporating the concepts of random molecular motion, concentration gradients, and entropy maximization. It’s not simply a matter of particles moving from high to low concentration; it's a consequence of the fundamental laws of physics driving systems towards a state of equilibrium. Understanding diffusion requires grasping its underlying mechanisms and appreciating its wide-ranging implications across various scientific disciplines. By combining different perspectives, we can develop a richer and more complete understanding of this ubiquitous process that shapes our world at every level, from the subatomic to the planetary. Further research and exploration of diffusion continue to reveal new insights and applications, highlighting its ongoing importance in scientific advancement and technological innovation.
Latest Posts
Latest Posts
-
Es Donde Pones La Cabeza Cuando Duermes
Apr 01, 2025
-
What Allows A Device To Be Managed Remotely
Apr 01, 2025
-
Identify The Parts Of The Sociological Definition Of Poverty
Apr 01, 2025
-
Managed Foodservice Differs From Commercial Foodservice In That
Apr 01, 2025
-
One Can Expect Their Sales Volume To Be
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Best Explains Diffusion . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
