Which Of The Following Statements About Enzymes Is Not True
Breaking News Today
Mar 21, 2025 · 6 min read
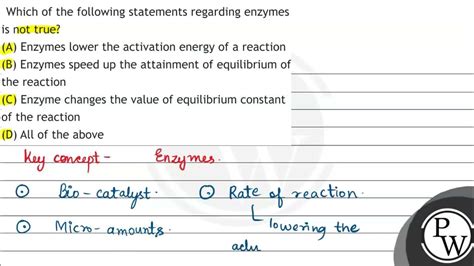
Table of Contents
Which of the following statements about enzymes is NOT true? A Comprehensive Exploration
Enzymes, the biological catalysts that drive the myriad chemical reactions within living organisms, are fascinating molecules. Understanding their properties and limitations is crucial in various fields, from medicine and biotechnology to environmental science. This article delves into common misconceptions surrounding enzymes, focusing on statements that are often incorrectly assumed to be true. We will dissect each statement, providing accurate information and highlighting the nuances of enzyme function.
Debunking Common Misconceptions about Enzymes
Let's address some frequently encountered statements about enzymes and determine which one is false. The key to understanding enzyme behavior lies in grasping their structure, function, and the factors that influence their activity.
Statement 1: Enzymes are consumed during a reaction.
FALSE. This is a pervasive misconception. Enzymes are not used up during the reactions they catalyze. They act by lowering the activation energy of a reaction, thereby speeding it up significantly. Once the reaction is complete, the enzyme remains unchanged and is free to catalyze the same reaction again and again. Think of them as efficient matchmakers – they bring reactants together, facilitating the reaction but not participating in it themselves. This remarkable property allows a small amount of enzyme to catalyze a vast number of reactions.
Statement 2: Enzymes increase the equilibrium constant of a reaction.
FALSE. Enzymes solely affect the rate of a reaction, not its equilibrium. The equilibrium constant (K<sub>eq</sub>) represents the ratio of products to reactants at equilibrium. Enzymes accelerate the forward and reverse reactions equally; thus, they do not alter the final equilibrium point. While they significantly speed up the attainment of equilibrium, the position of equilibrium remains the same. The enzyme merely provides an alternative pathway with lower activation energy, making the reaction faster, but the final equilibrium concentrations of reactants and products stay unchanged.
Statement 3: Enzyme activity is unaffected by temperature and pH.
FALSE. Enzyme activity is highly sensitive to both temperature and pH. Enzymes have optimal temperature and pH ranges, representing the conditions under which they function most efficiently. Outside of these ranges, their activity can be significantly reduced or even completely lost. High temperatures can denature enzymes, causing irreversible changes to their three-dimensional structure, leading to loss of function. Similarly, extreme pH values can disrupt the charges on amino acid side chains, affecting enzyme conformation and catalytic activity. The optimal temperature and pH are enzyme-specific, reflecting their evolutionary adaptation to their cellular environment.
Statement 4: All enzymes are proteins.
FALSE. While the vast majority of enzymes are proteins, this statement is not entirely accurate. A smaller group of enzymes are composed of catalytic RNA molecules, known as ribozymes. These RNA molecules exhibit catalytic activity, similar to protein enzymes, and play crucial roles in various biological processes, including RNA splicing and protein synthesis. The discovery of ribozymes challenged the long-held belief that only proteins could act as biological catalysts.
Statement 5: Enzymes are highly specific in their action.
TRUE. This is a fundamental characteristic of enzymes. They exhibit a remarkable degree of specificity, meaning that they typically catalyze only one specific type of reaction or a limited set of closely related reactions. This specificity stems from the precise three-dimensional structure of the enzyme, including the active site, which interacts with the substrate(s). The specific shape and chemical properties of the active site determine which substrates can bind and undergo catalysis. This high degree of specificity is essential for the precise control of metabolic pathways within living cells.
Statement 6: Enzyme activity is always linear with substrate concentration.
FALSE. Enzyme activity exhibits a characteristic pattern with respect to substrate concentration. At low substrate concentrations, the rate of reaction increases linearly with increasing substrate concentration. This is because the enzyme molecules are not saturated with substrate, and more substrate means more enzyme-substrate complexes forming. However, at high substrate concentrations, the rate of reaction plateaus. This is because the enzyme becomes saturated; all active sites are occupied, and further increases in substrate concentration cannot increase the reaction rate. This relationship is often described by the Michaelis-Menten kinetics, which demonstrates a hyperbolic curve rather than a linear one.
Statement 7: Enzyme inhibitors always permanently inactivate the enzyme.
FALSE. Enzyme inhibitors can be broadly classified into reversible and irreversible inhibitors. Irreversible inhibitors permanently modify the enzyme, rendering it inactive. This usually involves covalent modification of essential amino acid residues within the active site. In contrast, reversible inhibitors bind non-covalently to the enzyme, and the enzyme-inhibitor complex can dissociate, allowing the enzyme to regain its activity. Reversible inhibitors can be competitive (competing with the substrate for the active site) or non-competitive (binding to a site other than the active site, causing a conformational change that affects the enzyme's activity).
Statement 8: All enzymes require cofactors.
FALSE. While many enzymes require cofactors (non-protein components necessary for their activity), many others function perfectly well without them. Cofactors can be metal ions (e.g., zinc, magnesium) or organic molecules (coenzymes), often derived from vitamins. These cofactors can participate directly in the catalytic mechanism or contribute to the enzyme's stability and structure. The presence or absence of a cofactor requirement is a defining characteristic of different enzyme classes.
Statement 9: Enzymes work equally well under all conditions.
FALSE. As mentioned earlier, enzyme activity is greatly influenced by a variety of factors, including temperature, pH, substrate concentration, and the presence of inhibitors or activators. Optimal conditions for enzyme activity are specific to each enzyme and are crucial for its efficient functioning. Variations from optimal conditions can significantly reduce or eliminate enzyme activity, disrupting metabolic processes and potentially harming the organism. Understanding the optimal conditions for specific enzymes is crucial in various applications, such as industrial enzyme use and drug development.
Statement 10: Enzyme kinetics is solely determined by the enzyme's structure.
FALSE. While enzyme structure plays a pivotal role in defining its catalytic activity and specificity, enzyme kinetics is also influenced by other factors. The concentration of substrates and products, the presence of inhibitors or activators, temperature, pH, and ionic strength all contribute to the overall reaction rate. Understanding the interplay of these factors with enzyme structure is crucial for a complete understanding of enzyme kinetics and its regulation within living systems.
Conclusion: The Importance of Accurate Information
Understanding the intricacies of enzyme function is paramount in various scientific disciplines. The statements analyzed above highlight common misconceptions about enzymes. It’s crucial to appreciate the nuances of enzyme behavior, recognizing that their activity is highly dependent on various environmental factors and that not all enzymes behave identically. By accurately characterizing enzyme properties and function, we can better understand biological processes and leverage enzymes for various biotechnological and therapeutic applications. Continued research and exploration of enzyme activity continue to refine our knowledge and expand the potential applications of these remarkable biological catalysts.
Latest Posts
Latest Posts
-
Your Meeting Notes Are Unclassified This Means
Mar 28, 2025
-
Which Element Of A Story Is Most Clearly A Motif
Mar 28, 2025
-
The Sponsoring Of Scholarship By Turkic Dynasties
Mar 28, 2025
-
To Avoid A Spin While In A Skid You Should
Mar 28, 2025
-
The Designated Length Of A Ladder Is
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Statements About Enzymes Is Not True . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
