Why Is Evaporation Is A Cooling Process
Breaking News Today
Apr 01, 2025 · 5 min read
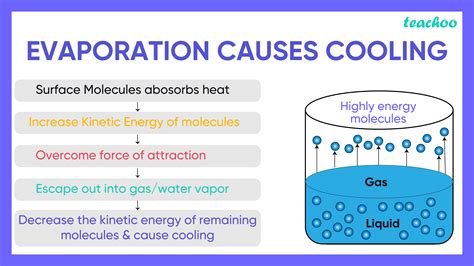
Table of Contents
Why is Evaporation a Cooling Process? Understanding the Science Behind Evaporative Cooling
Evaporation, the process where a liquid transforms into a gas, is a remarkably effective cooling mechanism. From sweating to industrial refrigeration, this natural phenomenon plays a crucial role in regulating temperature across various scales. But why exactly does evaporation lead to cooling? The answer lies in the interplay between molecular energy, heat transfer, and the properties of liquids and gases. This article delves into the scientific principles behind evaporative cooling, exploring its applications and providing a comprehensive understanding of this essential process.
Understanding the Kinetic Energy of Molecules
At the heart of evaporative cooling lies the concept of kinetic energy. Molecules within a liquid are constantly in motion, colliding with one another at varying speeds. These speeds, and consequently the kinetic energy, are distributed according to a Maxwell-Boltzmann distribution. This means there's a range of molecular speeds, with some molecules moving faster than others.
The Escape Velocity of Molecules
The molecules with the highest kinetic energy are located near the surface of the liquid. These high-energy molecules possess enough kinetic energy to overcome the attractive forces holding them within the liquid phase. This "escape velocity" allows them to break free from the liquid's surface and transition into the gaseous phase – a process we know as evaporation.
Heat Absorption: The Key to Cooling
The critical aspect of evaporative cooling lies in the energy required for the phase transition. When a molecule escapes the liquid, it carries away a significant amount of kinetic energy with it. This energy isn't simply lost; it's absorbed from the surrounding liquid. This absorption of energy manifests as a decrease in the average kinetic energy of the remaining molecules.
Lower Kinetic Energy Means Lower Temperature
A decrease in the average kinetic energy directly translates to a lower temperature. This is because temperature is a measure of the average kinetic energy of the molecules in a substance. Therefore, by removing high-energy molecules during evaporation, the remaining liquid experiences a net decrease in temperature – thus, the cooling effect.
Latent Heat of Vaporization: The Energy Thief
The amount of energy absorbed during evaporation is quantified by the latent heat of vaporization. This is the energy required to change one unit of mass of a liquid into its gaseous phase at a constant temperature. Water, for instance, has a relatively high latent heat of vaporization, meaning a significant amount of energy is needed to evaporate it. This is why water is such an effective coolant.
The Role of Intermolecular Forces
The strength of intermolecular forces within a liquid also influences the latent heat of vaporization. Stronger intermolecular forces require more energy to break, resulting in a higher latent heat of vaporization. This explains why substances with strong intermolecular forces, like water, exhibit a greater cooling effect upon evaporation compared to substances with weaker forces.
Environmental Factors Affecting Evaporation
Several environmental factors can influence the rate of evaporation and, consequently, the cooling effect. These include:
Temperature: A Higher Temperature, Faster Evaporation
Higher temperatures increase the average kinetic energy of the molecules, making it easier for them to escape the liquid's surface. This leads to faster evaporation and a more pronounced cooling effect.
Humidity: Less Evaporation in Humid Conditions
High humidity means the air already contains a significant amount of water vapor. This reduces the driving force for evaporation, as the air is less able to absorb more water molecules. Consequently, the cooling effect is less pronounced in humid environments.
Airflow: Increased Airflow, Enhanced Evaporation
Moving air removes water vapor from the vicinity of the liquid's surface, reducing the partial pressure of water vapor in the air. This creates a larger concentration gradient, accelerating evaporation and enhancing the cooling effect. This is why a breeze feels cool on a hot day.
Surface Area: More Surface Area, Faster Evaporation
A larger surface area exposes more liquid molecules to the atmosphere, increasing the number of molecules that can escape into the gaseous phase. This leads to faster evaporation and greater cooling.
Applications of Evaporative Cooling
Evaporative cooling finds numerous applications across diverse fields, including:
Human Body Thermoregulation: Sweating
Sweating is the body's natural mechanism for regulating temperature. As sweat evaporates from the skin's surface, it draws heat away, preventing overheating. This is particularly crucial during physical activity or in hot environments.
Industrial Refrigeration: Evaporative Condensers
Evaporative condensers are used in refrigeration systems to cool the refrigerant. These systems use water to cool the condenser, and the evaporation of water enhances the cooling process. This is more energy-efficient than traditional condenser systems.
HVAC Systems: Swamp Coolers
Swamp coolers, also known as evaporative air coolers, use water evaporation to cool air. They are particularly effective in dry climates, where the rapid evaporation of water provides substantial cooling.
Agriculture: Crop Cooling
Evaporative cooling can be used to mitigate the effects of high temperatures on crops, improving their growth and yield. Techniques like misting and sprinkler irrigation utilize evaporation to cool the surrounding air and reduce heat stress on plants.
Evaporative Cooling vs. Other Cooling Methods
Compared to other cooling methods like refrigeration, evaporative cooling offers several advantages:
- Energy Efficiency: It utilizes less energy than refrigeration systems, making it a more sustainable option.
- Environmental Friendliness: It doesn't rely on refrigerants that can harm the ozone layer or contribute to global warming.
- Simplicity and Cost-Effectiveness: Evaporative cooling systems are relatively simple and inexpensive to build and maintain.
However, evaporative cooling also has limitations:
- Effectiveness Depends on Humidity: Its effectiveness is significantly reduced in humid environments.
- Not Suitable for All Climates: It is most effective in dry and hot climates.
Conclusion: The Ubiquitous Cooling Power of Evaporation
Evaporation, a seemingly simple process, plays a vital role in countless natural and engineered systems. Its ability to cool is rooted in the fundamental principles of molecular kinetic energy, heat transfer, and phase transitions. Understanding these principles allows us to appreciate the significance of evaporative cooling in human physiology, industrial applications, and environmental processes. From the cooling effect of sweat to the functionality of sophisticated refrigeration systems, the phenomenon of evaporative cooling demonstrates the remarkable power of nature's ingenuity and its potential for sustainable solutions. Further research into optimizing and expanding the applications of evaporative cooling holds significant promise for addressing the challenges of energy efficiency and environmental sustainability in a warming world.
Latest Posts
Latest Posts
-
Amoeba Sisters Video Recap Dna Replication Answer Key
Apr 02, 2025
-
Tu Deseas Mirar Cuadros Paintings De Picasso El Arte
Apr 02, 2025
-
Skin Inflammations That Increase In Frequency With Age
Apr 02, 2025
-
Florida Life Insurance Exam Questions And Answers Pdf
Apr 02, 2025
-
A Tact Is A Procedure Short For Tactic
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Why Is Evaporation Is A Cooling Process . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
