A Dendritic Or Langerhans Cell Is A Specialized ________.
Breaking News Today
Mar 16, 2025 · 7 min read
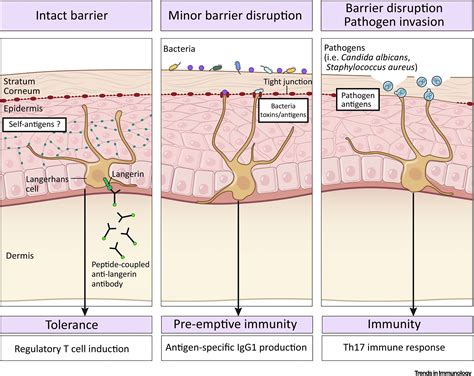
Table of Contents
A Dendritic Cell or Langerhans Cell is a Specialized Antigen-Presenting Cell
A dendritic cell (DC) or Langerhans cell (LC) is a specialized antigen-presenting cell (APC). These immune system sentinels play a crucial role in initiating and modulating adaptive immune responses. Their unique morphology, strategic location, and potent antigen-presenting capabilities make them vital players in the intricate dance of immunity. This article will delve deep into the characteristics, functions, and significance of these specialized cells, exploring their role in both health and disease.
Understanding the Morphology and Location of Dendritic Cells and Langerhans Cells
Dendritic cells are characterized by their distinctive, branched morphology – hence the name "dendritic," meaning tree-like. These projections, or dendrites, significantly increase their surface area, maximizing their capacity to capture antigens. While the general structure is similar, different subsets of dendritic cells exhibit variations in their morphology depending on their maturation state and tissue location.
Langerhans Cells: The Skin's Guardians
Langerhans cells, a specific type of dendritic cell, are predominantly found in the epidermis, the outermost layer of the skin. They form a crucial first line of defense against pathogens that attempt to breach the skin barrier. Their strategic location allows them to swiftly detect invading microorganisms and initiate an immune response. These cells are easily identifiable under a microscope due to their characteristic Birbeck granules, which are unique intracellular organelles of unknown function.
Other Dendritic Cell Subsets and Their Locations
Beyond Langerhans cells, a diverse array of dendritic cell subsets exists, each with specific anatomical locations and functions. These include:
- Conventional dendritic cells (cDCs): These are found in various tissues, including the spleen, lymph nodes, and lymphoid organs. They are further subdivided into cDC1 and cDC2 subsets, which differ in their antigen-presenting capabilities and the types of T cells they activate. cDC1s are crucial for activating cytotoxic T lymphocytes (CTLs), while cDC2s are better at activating helper T lymphocytes (Th cells).
- Plasmacytoid dendritic cells (pDCs): These cells primarily reside in the blood and lymphoid tissues. They are specialized in producing type I interferons (IFNs), which are potent antiviral molecules playing a critical role in the innate immune response against viral infections.
- Follicular dendritic cells (FDCs): Unlike other dendritic cells, FDCs are not of hematopoietic origin. They reside within the follicles of secondary lymphoid organs like lymph nodes and spleen, presenting antigens to B cells to facilitate antibody production.
The Crucial Role of Dendritic Cells in Antigen Presentation
The primary function of dendritic cells and Langerhans cells lies in their ability to present antigens to T lymphocytes, initiating the adaptive immune response. This process involves several critical steps:
1. Antigen Capture and Processing
Dendritic cells use various mechanisms to capture antigens:
- Phagocytosis: The engulfment of pathogens and cellular debris.
- Pinocytosis: The uptake of fluids and dissolved substances.
- Receptor-mediated endocytosis: The targeted uptake of antigens bound to specific receptors on the cell surface.
Once captured, the antigen is processed within the dendritic cell. This involves breaking down the antigen into smaller peptide fragments that can be presented to T cells.
2. MHC Presentation
Processed antigen peptides are then loaded onto major histocompatibility complex (MHC) molecules. There are two main classes of MHC molecules:
- MHC class I: Presents peptides derived from intracellular pathogens to CD8+ T cells (cytotoxic T lymphocytes), which are responsible for killing infected cells.
- MHC class II: Presents peptides derived from extracellular pathogens to CD4+ T cells (helper T lymphocytes), which coordinate various aspects of the immune response.
3. T Cell Activation
The dendritic cell, now bearing MHC-peptide complexes, migrates to secondary lymphoid organs, such as lymph nodes. Here, it interacts with T cells. If a T cell receptor (TCR) recognizes the presented peptide, it triggers T cell activation. This involves a complex series of signaling events that lead to T cell proliferation and differentiation into effector cells capable of combating the infection. This process is essential for both cell-mediated and humoral immunity.
Dendritic Cells in Immune Regulation: Beyond Antigen Presentation
Dendritic cells are not merely passive antigen presenters; they actively shape the immune response. Their ability to polarize T cells into different subsets, such as Th1, Th2, Th17, and Treg cells, is vital for determining the nature and intensity of the immune response. This regulatory function ensures an appropriate immune response to a specific threat, preventing overreaction and promoting immune homeostasis. For example, they can promote tolerance to self-antigens, preventing autoimmune diseases.
The Significance of Co-stimulatory Molecules
The activation of T cells is dependent on not only MHC-peptide presentation but also the presence of co-stimulatory molecules on the surface of the dendritic cell. These molecules, such as CD80 and CD86, bind to corresponding receptors on the T cell, providing a crucial second signal that enhances T cell activation. The absence of co-stimulation can lead to T cell anergy or tolerance, preventing an unwanted immune response.
Cytokine Production and Modulation
Dendritic cells produce a diverse array of cytokines, which are signaling molecules that influence the development and function of other immune cells. These cytokines play a significant role in determining the type of immune response generated. For instance, the production of IL-12 by dendritic cells promotes the development of Th1 cells, which are crucial for combating intracellular pathogens. In contrast, IL-4 promotes Th2 cell development, important for combating extracellular parasites and helminths. The balance of cytokine production by dendritic cells is crucial for maintaining immune homeostasis and tailoring the response to specific threats.
Dendritic Cells in Health and Disease
The role of dendritic cells extends far beyond the realm of basic immunology. Their dysfunction is implicated in a wide array of diseases:
Autoimmune Diseases
Dysregulation of dendritic cells, leading to aberrant activation of self-reactive T cells, is a key factor in the pathogenesis of autoimmune diseases such as rheumatoid arthritis, multiple sclerosis, and type 1 diabetes.
Cancer
Dendritic cells play a dual role in cancer: They can initiate anti-tumor immunity by presenting tumor antigens to T cells, but they can also promote tumor growth and metastasis by suppressing anti-tumor immune responses. The capacity of tumors to suppress dendritic cell function is a significant challenge in cancer immunotherapy.
Infections
The ability of pathogens to evade or manipulate dendritic cell function is a major factor in their ability to cause disease. Some viruses, for example, actively inhibit dendritic cell maturation and antigen presentation, hindering the immune system's ability to effectively combat the infection.
Allergic Diseases
Dendritic cells play a critical role in the development of allergic diseases by presenting allergens to T cells, promoting the development of Th2 cells and the production of IgE antibodies.
Therapeutic Applications Targeting Dendritic Cells
Given their central role in immunity, dendritic cells are attractive targets for therapeutic interventions. These include:
Dendritic Cell-Based Vaccines
Dendritic cells can be used to create vaccines that are highly effective at stimulating anti-tumor or anti-viral immunity. These vaccines involve loading dendritic cells with tumor antigens or viral peptides in vitro before administering them to patients, thereby stimulating a targeted immune response.
Immunotherapy Strategies
Various immunotherapy strategies aim to modulate dendritic cell function to enhance anti-tumor immunity or suppress aberrant immune responses in autoimmune diseases.
Future Directions
Ongoing research continues to unravel the intricacies of dendritic cell biology and their role in health and disease. This research holds immense promise for developing innovative therapies for a wide range of conditions, leveraging the potent immunoregulatory capabilities of these remarkable cells.
Conclusion
The dendritic cell or Langerhans cell, as a specialized antigen-presenting cell, stands as a cornerstone of the adaptive immune system. Their unique morphology, strategic location, and ability to capture, process, and present antigens, combined with their capacity to regulate immune responses, are crucial for maintaining immune homeostasis and combating infections and diseases. Further understanding of their complex functions will undoubtedly lead to breakthroughs in developing effective immunotherapies for various conditions. Their importance extends far beyond their initial identification and continues to expand as research uncovers new layers of complexity and therapeutic potential. The dendritic cell, in all its specialized forms, remains a fascinating and vital component of our immune defense.
Latest Posts
Latest Posts
-
Cdl Combination Test Questions And Answers Pdf
Mar 18, 2025
-
Life Insurance Exam Questions And Answers Pdf
Mar 18, 2025
-
The Direct Carry Is Used To Transfer A Patient
Mar 18, 2025
-
The Emancipation Proclamation Of January 1 1863 Quizlet
Mar 18, 2025
-
These Cards Will Get You Drunk Quizlet
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about A Dendritic Or Langerhans Cell Is A Specialized ________. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
