Chemical Reactions Can Be Classified Based On Changes In Chemical
Breaking News Today
Mar 16, 2025 · 6 min read
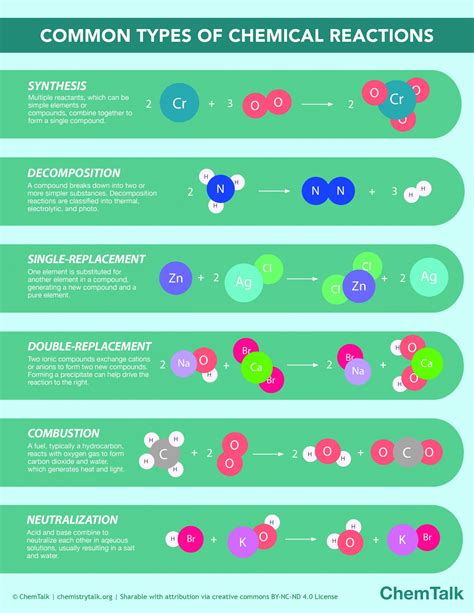
Table of Contents
Chemical Reactions: A Classification Based on Chemical Changes
Chemical reactions are the foundation of chemistry, representing the dynamic transformations of matter. Understanding how these reactions occur and how they can be categorized is crucial for comprehending the world around us, from the rusting of iron to the processes within our own bodies. This article delves into the classification of chemical reactions based on the changes in chemical composition and energy involved. We'll explore various reaction types, their characteristics, and real-world examples, providing a comprehensive overview of this fundamental aspect of chemistry.
Categorizing Chemical Reactions: A Multifaceted Approach
Chemical reactions aren't just random events; they follow specific patterns and can be classified based on several criteria. While many reactions exhibit characteristics of multiple categories, a primary classification focuses on the changes in chemical composition:
1. Combination Reactions (Synthesis Reactions):
Definition: In a combination reaction, two or more reactants combine to form a single, more complex product. This process typically involves the formation of new chemical bonds.
General Form: A + B → AB
Examples:
- Formation of water: 2H₂ + O₂ → 2H₂O Two molecules of hydrogen gas combine with one molecule of oxygen gas to produce two molecules of water. This is a highly exothermic reaction, releasing significant heat.
- Formation of magnesium oxide: 2Mg + O₂ → 2MgO Magnesium reacts vigorously with oxygen in the air, producing magnesium oxide and releasing considerable heat and light.
- Formation of iron(III) oxide: 4Fe + 3O₂ → 2Fe₂O₃ Iron rusting is a classic example of a combination reaction, where iron reacts slowly with oxygen to form iron(III) oxide (rust). This is an oxidation-reduction reaction, which we will discuss further below.
2. Decomposition Reactions:
Definition: A decomposition reaction is the opposite of a combination reaction. A single compound breaks down into two or more simpler substances. This often requires energy input, such as heat or electricity.
General Form: AB → A + B
Examples:
- Electrolysis of water: 2H₂O → 2H₂ + O₂ Passing an electric current through water decomposes it into hydrogen and oxygen gases.
- Thermal decomposition of calcium carbonate: CaCO₃ → CaO + CO₂ Heating calcium carbonate (limestone) causes it to decompose into calcium oxide (quicklime) and carbon dioxide gas. This is used extensively in the production of cement.
- Decomposition of hydrogen peroxide: 2H₂O₂ → 2H₂O + O₂ Hydrogen peroxide spontaneously decomposes into water and oxygen gas, although this process can be accelerated by catalysts.
3. Single Displacement Reactions (Substitution Reactions):
Definition: In a single displacement reaction, a more reactive element replaces a less reactive element in a compound. This is a redox reaction, involving the transfer of electrons.
General Form: A + BC → AC + B
Examples:
- Reaction of zinc with hydrochloric acid: Zn + 2HCl → ZnCl₂ + H₂ Zinc, being more reactive than hydrogen, displaces hydrogen from hydrochloric acid, forming zinc chloride and hydrogen gas.
- Reaction of iron with copper(II) sulfate: Fe + CuSO₄ → FeSO₄ + Cu Iron replaces copper in copper(II) sulfate solution, forming iron(II) sulfate and depositing copper metal. This demonstrates the relative reactivity of iron and copper.
- Reaction of sodium with water: 2Na + 2H₂O → 2NaOH + H₂ Sodium, a highly reactive alkali metal, displaces hydrogen from water, forming sodium hydroxide and releasing hydrogen gas. This reaction is highly exothermic.
4. Double Displacement Reactions (Metathesis Reactions):
Definition: A double displacement reaction involves the exchange of ions between two compounds, typically in an aqueous solution. This often results in the formation of a precipitate, a gas, or water.
General Form: AB + CD → AD + CB
Examples:
- Precipitation of silver chloride: AgNO₃ + NaCl → AgCl + NaNO₃ Silver nitrate reacts with sodium chloride to form a white precipitate of silver chloride and soluble sodium nitrate.
- Neutralization reaction: HCl + NaOH → NaCl + H₂O Hydrochloric acid reacts with sodium hydroxide to form sodium chloride (salt) and water. This is an acid-base reaction, a specific type of double displacement reaction.
- Formation of a gas: Na₂CO₃ + 2HCl → 2NaCl + H₂O + CO₂ Sodium carbonate reacts with hydrochloric acid to produce sodium chloride, water, and carbon dioxide gas.
5. Combustion Reactions:
Definition: Combustion reactions involve the rapid reaction of a substance with oxygen, typically producing heat and light. This is a highly exothermic redox reaction.
General Form: Fuel + O₂ → Products (usually CO₂, H₂O)
Examples:
- Burning of methane: CH₄ + 2O₂ → CO₂ + 2H₂O Methane, the primary component of natural gas, burns in oxygen to produce carbon dioxide and water.
- Burning of propane: C₃H₈ + 5O₂ → 3CO₂ + 4H₂O Propane, a common fuel, undergoes combustion to yield carbon dioxide and water.
- Burning of wood: Wood (cellulose, etc.) + O₂ → CO₂, H₂O, ash The complex organic compounds in wood react with oxygen during combustion, producing carbon dioxide, water, and ash.
6. Redox Reactions (Oxidation-Reduction Reactions):
Definition: Redox reactions involve the transfer of electrons between reactants. One species undergoes oxidation (loss of electrons), while another undergoes reduction (gain of electrons). Many of the reaction types described above are redox reactions.
Examples:
- Rusting of iron (already mentioned above): This involves the oxidation of iron (Fe → Fe³⁺ + 3e⁻) and the reduction of oxygen (O₂ + 4e⁻ → 2O²⁻).
- Combustion reactions (already mentioned above): The fuel is oxidized, and oxygen is reduced.
- Single displacement reactions (already mentioned above): The more reactive element is oxidized, and the less reactive element in the compound is reduced.
Beyond Basic Classifications: A Deeper Dive into Reaction Mechanisms
While the categories above provide a useful framework, understanding chemical reactions requires delving into their mechanisms – the step-by-step processes involved. Factors like reaction rates, activation energies, catalysts, and reaction intermediates significantly influence the course of a reaction.
Reaction Rates and Equilibrium:
The rate of a chemical reaction describes how quickly reactants are consumed and products are formed. Several factors influence reaction rates, including:
- Concentration of reactants: Higher concentrations generally lead to faster reaction rates.
- Temperature: Increasing temperature typically increases reaction rates.
- Surface area: For reactions involving solids, a larger surface area increases the rate.
- Presence of a catalyst: Catalysts accelerate reaction rates without being consumed in the process.
Reactions often reach a state of equilibrium, where the rate of the forward reaction equals the rate of the reverse reaction. The equilibrium constant (K) expresses the relative amounts of reactants and products at equilibrium.
Activation Energy and Reaction Mechanisms:
The activation energy (Ea) is the minimum energy required for a reaction to occur. Reaction mechanisms describe the sequence of elementary steps involved in a reaction, providing insights into how reactants transform into products. Understanding reaction mechanisms is crucial for designing and controlling chemical processes.
Real-World Applications: The Importance of Understanding Chemical Reactions
The principles of chemical reactions are fundamental to numerous fields:
- Industrial Chemistry: Chemical reactions are the basis for synthesizing countless products, from pharmaceuticals and plastics to fertilizers and fuels.
- Environmental Science: Understanding chemical reactions is crucial for addressing environmental challenges, such as pollution control and remediation.
- Biochemistry: Biological processes rely on a vast array of chemical reactions, forming the foundation of life itself.
- Materials Science: Developing new materials with desired properties involves careful control of chemical reactions.
- Medicine: Drug discovery and development rely heavily on understanding chemical reactions and their effects on the body.
Conclusion: A Dynamic and Ever-Evolving Field
The classification of chemical reactions based on changes in chemical composition provides a valuable framework for understanding the diverse transformations of matter. However, this is just the beginning. Delving into reaction mechanisms, kinetics, and thermodynamics provides a deeper appreciation of the intricate processes involved. As our understanding of chemical reactions continues to evolve, so too will our ability to harness their power for the benefit of society. The study of chemical reactions is a dynamic and ever-evolving field, crucial for advancements in various scientific and technological domains. Continuous research and exploration will further illuminate the complexities and applications of this fundamental aspect of chemistry.
Latest Posts
Latest Posts
-
If An Individual Is Heterozygous For A Particular Trait
Mar 18, 2025
-
If You Add More Enzyme The Reaction Will
Mar 18, 2025
-
The Purpose Of A Hazcom Program Is To Ensure That
Mar 18, 2025
-
Describe The Continuous Nature Of The Physical Fitness Concept
Mar 18, 2025
-
High Levels Of Cholesterol Can First Lead Directly To
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Chemical Reactions Can Be Classified Based On Changes In Chemical . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
