Continuing Review Of An Approved And Ongoing Study Posing
Breaking News Today
Mar 12, 2025 · 6 min read
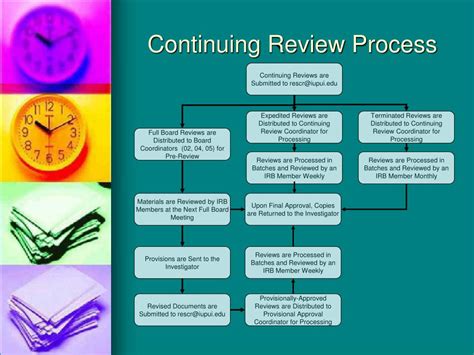
Table of Contents
Continuing Review of an Approved and Ongoing Study: A Comprehensive Guide
The ethical conduct of research involving human subjects is paramount. Once a study receives initial approval from an Institutional Review Board (IRB), the journey doesn't end. Ongoing research requires continuous monitoring and evaluation through a process known as continuing review. This process ensures the continued protection of participants, the integrity of the research, and adherence to regulatory guidelines. This article delves into the intricacies of continuing review, providing a comprehensive understanding for researchers and IRB members alike.
Understanding the Importance of Continuing Review
Continuing review is not merely a bureaucratic hurdle; it's a critical safeguard for human subjects research. Its importance stems from several key factors:
1. Protecting Participants' Rights and Welfare:
The primary goal of continuing review is to protect the rights and welfare of research participants. Over time, unforeseen risks or problems might emerge, requiring adjustments to the study protocol. Continuing review allows for prompt identification and mitigation of these risks, preventing harm to participants. This includes monitoring for adverse events, assessing the effectiveness of informed consent procedures, and ensuring ongoing participant safety.
2. Ensuring Data Integrity and Scientific Validity:
Continuing review also contributes to maintaining the integrity and scientific validity of the research. By regularly evaluating the study's progress, methodology, and data collection techniques, IRBs can identify any deviations from the approved protocol or potential biases that could compromise the results. This ensures that the research remains robust and reliable.
3. Adherence to Regulatory Compliance:
Continuing review is mandated by federal regulations and guidelines, such as those outlined by the U.S. Department of Health and Human Services (HHS). Failing to conduct appropriate continuing review can result in severe penalties, including suspension of funding and legal ramifications. Compliance is not simply a legal obligation; it reflects a commitment to ethical research practices.
The Continuing Review Process: A Step-by-Step Guide
The specifics of the continuing review process may vary slightly depending on the IRB and the type of research, but the general steps are consistent:
1. Frequency of Continuing Review:
The frequency of continuing review is determined by the IRB, considering the nature and risk level of the study. Generally, studies involving minimal risk may require review annually, while those with higher risk levels may necessitate more frequent reviews, potentially every six months or even more often. Studies involving vulnerable populations often require more stringent and frequent review.
2. Submission of Continuing Review Materials:
Researchers are typically required to submit a continuing review application to the IRB, which usually includes:
- Progress Report: A detailed report outlining the progress of the study, including the number of participants enrolled, data collected, and any significant findings.
- Adverse Event Reports: Documentation of any adverse events experienced by participants, along with actions taken to address them.
- Modifications to the Protocol: Any changes made to the research protocol since the last review, including changes to the consent form, procedures, or data collection methods. Even seemingly minor modifications must be reported and approved.
- Participant Recruitment and Retention Rates: An update on the progress of participant recruitment and retention, noting any challenges encountered.
- Data Analysis Plan: A description of the planned data analysis, highlighting any changes or refinements.
- Certification of Continuing Compliance: A statement from the principal investigator certifying that the study is being conducted in accordance with the approved protocol and regulations.
3. IRB Review and Decision:
The IRB reviews the continuing review application meticulously, assessing the continued protection of participants, the scientific integrity of the study, and adherence to regulatory guidelines. The IRB may request clarifications or additional information from the researcher. The IRB will then issue a decision, which may include:
- Approval: The study is permitted to continue as planned.
- Approval with Modifications: The study may continue, but with specified changes to the protocol or procedures.
- Disapproval: The study is terminated due to ethical concerns or regulatory violations.
4. Addressing IRB Concerns:
If the IRB identifies any concerns during the continuing review process, researchers must address these concerns promptly and thoroughly. This may involve revising the research protocol, implementing additional safeguards, or providing further documentation. Failure to adequately address IRB concerns can lead to the suspension or termination of the study.
Specific Considerations in Continuing Review
Several factors warrant special attention during the continuing review process:
1. Unforeseen Risks and Adverse Events:
Prompt reporting of any unforeseen risks or adverse events is crucial. Researchers must meticulously document and analyze these events, taking appropriate measures to protect participants and mitigate future risks. The IRB will assess the severity and implications of any reported events.
2. Changes in the Research Protocol:
Any changes to the research protocol, no matter how minor, must be submitted to the IRB for review and approval before implementation. This includes changes to the consent form, inclusion/exclusion criteria, data collection methods, or study procedures. Failure to obtain approval for protocol changes can invalidate the research and compromise the integrity of the study.
3. Data Security and Privacy:
Continuing review should also address the security and privacy of participant data. Researchers must demonstrate that appropriate measures are in place to protect the confidentiality and security of data, adhering to all relevant data protection regulations (such as HIPAA). The IRB will evaluate the adequacy of data security measures throughout the continuing review process.
4. Vulnerable Populations:
Studies involving vulnerable populations (e.g., children, pregnant women, prisoners) require particularly rigorous continuing review, with a greater emphasis on participant protection and informed consent procedures. The IRB will scrutinize these studies carefully, ensuring that additional safeguards are in place to address potential risks.
5. Longitudinal Studies:
Longitudinal studies, which follow participants over extended periods, present unique challenges for continuing review. Researchers must address the potential for changes in participants' circumstances, such as changes in health status or contact information, and the need for ongoing informed consent. Maintaining participant engagement and minimizing attrition are critical aspects of continuing review for longitudinal studies.
The Role of the Principal Investigator (PI)
The PI plays a vital role in the continuing review process. Their responsibilities include:
- Accurate and Timely Reporting: Providing accurate and timely reports to the IRB, accurately reflecting the progress of the study and any significant events.
- Proactive Risk Management: Proactively identifying and mitigating potential risks to participants.
- Compliance with Regulations: Ensuring that the study is conducted in full compliance with all relevant regulations and guidelines.
- Addressing IRB Concerns: Promptly addressing any concerns raised by the IRB, implementing necessary changes to protect participants and maintain the integrity of the study.
- Maintaining Accurate Records: Maintaining accurate and complete records of all aspects of the study, including participant data, adverse events, and protocol modifications.
Conclusion
Continuing review of approved and ongoing studies is an indispensable element of ethical human subjects research. It provides a critical mechanism for protecting participants, maintaining data integrity, and ensuring compliance with regulatory guidelines. By meticulously following the continuing review process, researchers contribute to the ethical advancement of scientific knowledge and uphold the highest standards of research integrity. The collaborative effort of researchers and IRBs is key to successful and ethical human subjects research, fostering trust and ensuring the well-being of all involved. A thorough understanding of continuing review procedures is not just a matter of compliance; it is a fundamental commitment to responsible and ethical research.
Latest Posts
Latest Posts
-
Management Is Defined As The Pursuit Of Organizational Goals
Mar 12, 2025
-
When Communicating With A Visually Impaired Patient You Should
Mar 12, 2025
-
The First Step In Creating A Public Policy Involves
Mar 12, 2025
-
Normally Sodium And Potassium Leakage Channels Differ Because
Mar 12, 2025
-
What Is The Purpose Of Windows Sandbox
Mar 12, 2025
Related Post
Thank you for visiting our website which covers about Continuing Review Of An Approved And Ongoing Study Posing . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
