First 36 Elements Of The Periodic Table
Breaking News Today
Apr 01, 2025 · 7 min read
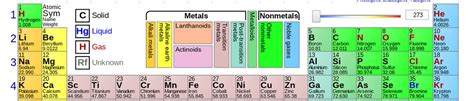
Table of Contents
The First 36 Elements: A Deep Dive into the Building Blocks of Matter
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic number and recurring chemical properties. Understanding the first 36 elements is crucial for grasping fundamental chemical principles and their applications in various fields. This comprehensive guide delves into the properties, uses, and significance of these foundational elements, providing a detailed look at their individual characteristics and interrelationships.
The First 18 Elements: The Foundation of Chemistry
The first 18 elements lay the groundwork for understanding the periodic table's structure and the behavior of matter. These elements span the first three rows and represent a diverse range of properties, from the inert gases to highly reactive metals and nonmetals.
Row 1: Hydrogen and Helium – The Simplest Atoms
-
Hydrogen (H): The most abundant element in the universe, hydrogen is a lightweight gas with a single proton and electron. Its versatility is evident in its diverse applications, including fuel cells (hydrogen fuel technology), ammonia production (Haber-Bosch process), and various industrial processes. Its isotope, deuterium, plays a vital role in nuclear fusion research.
-
Helium (He): A noble gas characterized by its inertness and low density. Helium's low reactivity makes it invaluable in applications requiring an inert atmosphere, such as welding and cryogenics (liquid helium for MRI machines). Its lightness also makes it useful in balloons and airships.
Row 2: Lithium to Neon – Introducing the Alkali Metals and Halogens
Row 2 introduces more complex atoms, showcasing the emergence of distinct chemical families.
-
Lithium (Li): An alkali metal, lithium is known for its low density and high reactivity. It finds uses in rechargeable batteries (lithium-ion batteries), lubricating greases, and psychiatric medications.
-
Beryllium (Be): An alkaline earth metal, beryllium possesses high strength and stiffness, making it valuable in aerospace applications and specialized tools. However, it is also toxic.
-
Boron (B): A metalloid, boron exhibits properties of both metals and nonmetals. It's a key component of borax and is crucial in various industrial applications including glass manufacturing (borosilicate glass).
-
Carbon (C): The fundamental building block of life, carbon forms an incredible variety of compounds due to its ability to bond in numerous ways. It is present in all organic molecules and forms the basis of organic chemistry. Its allotropes, diamond and graphite, display vastly different properties.
-
Nitrogen (N): A crucial component of the Earth's atmosphere, nitrogen forms a diatomic molecule (N₂) that is relatively unreactive. It's essential for plant growth (nitrogen fertilizers) and is used in the production of ammonia and other compounds.
-
Oxygen (O): Essential for respiration in most living organisms, oxygen is highly reactive and supports combustion. It plays a critical role in various biochemical processes and industrial applications.
-
Fluorine (F): A highly reactive halogen, fluorine is known for its electronegativity. It's used in various industrial applications, including the production of fluorocarbons (Teflon).
-
Neon (Ne): A noble gas, neon is known for its distinctive red glow when electrified, making it useful in signage and lighting.
Row 3: Sodium to Argon – Expanding Chemical Families
Row 3 further expands on the trends established in the previous rows, introducing elements with increasing atomic complexity.
-
Sodium (Na): An alkali metal, sodium is highly reactive and essential for biological processes. It's widely used in the production of table salt (sodium chloride) and various industrial chemicals.
-
Magnesium (Mg): An alkaline earth metal, magnesium is a lightweight and strong metal used in various alloys (magnesium alloys for aerospace). It also plays an important role in biological systems.
-
Aluminum (Al): A highly abundant metal, aluminum is known for its lightness and corrosion resistance. It's extensively used in packaging, transportation (aluminum cans and aircraft parts), and construction.
-
Silicon (Si): A metalloid, silicon is the second most abundant element in the Earth's crust. It's crucial in the semiconductor industry (silicon chips), glass manufacturing, and the production of silicones.
-
Phosphorus (P): A nonmetal, phosphorus is essential for life, playing a crucial role in DNA and energy transfer. It's used in fertilizers and various industrial applications.
-
Sulfur (S): A nonmetal, sulfur is found in various minerals and is used in the production of sulfuric acid (sulfuric acid in batteries and fertilizers) and other important chemicals.
-
Chlorine (Cl): A halogen, chlorine is a highly reactive element used in water purification (chlorinated water) and various industrial processes.
-
Argon (Ar): A noble gas, argon is used in welding and other applications requiring an inert atmosphere.
The Next 18 Elements: Delving Deeper into Chemical Complexity
Elements 19-36 introduce transition metals, adding another layer of complexity to the periodic table. These elements exhibit a diverse range of oxidation states and complex chemical behavior.
Transition Metals: Introducing d-block Elements
The transition metals, spanning elements 21-30, showcase the filling of the d-orbitals, leading to variable oxidation states and the formation of colorful compounds.
-
Potassium (K): An alkali metal, potassium is crucial for biological functions and is used in fertilizers and various industrial applications.
-
Calcium (Ca): An alkaline earth metal, calcium is a vital component of bones and teeth. It is also used in various industrial applications.
-
Scandium (Sc): A transition metal, scandium is used in high-intensity lighting and some alloys.
-
Titanium (Ti): A strong and lightweight transition metal, titanium is highly corrosion-resistant, making it valuable in aerospace, medical implants (titanium implants), and other applications.
-
Vanadium (V): A transition metal used in various alloys, including high-speed steel.
-
Chromium (Cr): A transition metal known for its high corrosion resistance, chromium is used in stainless steel (chromium in stainless steel) and various plating processes.
-
Manganese (Mn): A transition metal used in steel alloys and as a component of various catalysts.
-
Iron (Fe): One of the most abundant and important transition metals, iron is essential for oxygen transport in blood (hemoglobin) and is extensively used in steel production (iron in steel production).
-
Cobalt (Co): A transition metal used in alloys, magnets (cobalt magnets), and certain catalysts.
-
Nickel (Ni): A transition metal used in various alloys, including stainless steel, and as a catalyst.
-
Copper (Cu): A transition metal known for its excellent electrical conductivity, copper is used in electrical wiring, plumbing, and various alloys (brass and bronze).
-
Zinc (Zn): A transition metal, zinc is essential for many biological functions and is used in galvanizing (zinc coating to prevent corrosion).
Expanding the Nonmetals and Metalloids
Elements beyond the transition metals further showcase the diverse properties of nonmetals and metalloids.
-
Gallium (Ga): A metalloid with a low melting point, gallium is used in semiconductors and various electronic applications.
-
Germanium (Ge): A metalloid used in semiconductors and fiber optics.
-
Arsenic (As): A metalloid, arsenic is toxic and has limited industrial applications.
-
Selenium (Se): A nonmetal, selenium is an essential trace element for various biological functions.
-
Bromine (Br): A halogen, bromine is a reddish-brown liquid used in various industrial processes.
-
Krypton (Kr): A noble gas, krypton is used in some lighting applications.
The Significance of Understanding the First 36 Elements
Understanding the first 36 elements is critical for numerous reasons:
-
Foundation of Chemistry: These elements form the basis for understanding fundamental chemical concepts like bonding, reactivity, and periodic trends.
-
Industrial Applications: Many of these elements are crucial for various industrial processes, including manufacturing, energy production, and materials science.
-
Biological Importance: Several of these elements are essential for biological processes, playing vital roles in metabolism and overall health.
-
Technological Advancements: These elements are key components in numerous technologies, including electronics, medicine, and energy production.
-
Environmental Impact: Understanding the properties and behavior of these elements is crucial for assessing and mitigating their environmental impact.
This detailed overview of the first 36 elements provides a strong foundation for further exploration of the periodic table and its immense significance in the world around us. Further research into specific elements and their applications can deepen your understanding of their individual importance and their interconnected roles in the natural world and technological advancements.
Latest Posts
Latest Posts
-
Which Below Are Steps In Effective Inspections
Apr 02, 2025
-
What Are The Two Collisions That Happen In A Crash
Apr 02, 2025
-
A Lucky Individual Won The State Lottery
Apr 02, 2025
-
Correctly Label The Following Anatomical Parts Of A Kidney
Apr 02, 2025
-
Continuously Learning About Your Captivity Environment And The Captor
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about First 36 Elements Of The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
