First Ten Elements On The Period Table
Breaking News Today
Mar 19, 2025 · 7 min read
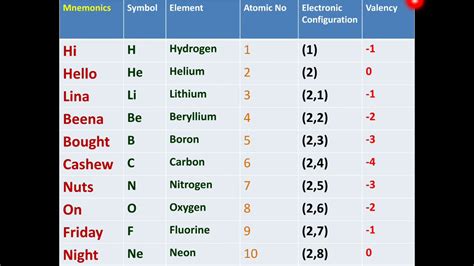
Table of Contents
The First Ten Elements: A Deep Dive into the Building Blocks of Matter
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. Understanding the first ten elements – Hydrogen (H), Helium (He), Lithium (Li), Beryllium (Be), Boron (B), Carbon (C), Nitrogen (N), Oxygen (O), Fluorine (F), and Neon (Ne) – provides a fundamental grasp of chemical behavior and the building blocks of the universe. This comprehensive guide delves into each element, exploring their properties, applications, and significance in the larger context of chemistry and beyond.
Hydrogen (H): The Simplest and Most Abundant
Hydrogen, the first element on the periodic table, is the simplest and most abundant element in the universe. Its atom consists of just one proton and one electron. This simplicity belies its importance.
Properties of Hydrogen:
- Gaseous State: Hydrogen exists as a diatomic gas (H₂) under standard conditions.
- Highly Reactive: It readily reacts with many elements, forming numerous compounds. This reactivity stems from its single electron, readily participating in chemical bonds.
- Isotopes: Hydrogen has three isotopes: protium (¹H), deuterium (²H or D), and tritium (³H or T), differing in the number of neutrons.
- Colorless and Odorless: In its pure form, hydrogen gas is colorless and odorless.
Applications of Hydrogen:
- Ammonia Production (Haber-Bosch process): A cornerstone of fertilizer production, this process utilizes hydrogen to create ammonia (NH₃).
- Fuel: Hydrogen is considered a clean fuel source, producing only water as a byproduct when burned. However, challenges remain in efficient storage and transportation.
- Metal Refining: Hydrogen is used in the refining of metals, reducing metal oxides to their pure forms.
- Chemical Industry: Hydrogen serves as a crucial reactant in numerous chemical processes.
Helium (He): An Inert Noble Gas
Helium, the second element, is a noble gas, meaning it's exceptionally unreactive due to its full electron shell. Its unique properties make it invaluable in various applications.
Properties of Helium:
- Inert: Its full electron shell renders it chemically inert, making it non-reactive with other elements.
- Low Density: Helium is the second-lightest element, significantly lighter than air, allowing it to float.
- Low Boiling Point: Helium has the lowest boiling point of all elements, making it useful in cryogenics.
- Colorless and Odorless: Like hydrogen, it's colorless and odorless in its gaseous state.
Applications of Helium:
- Balloons and Airships: Its low density makes it ideal for inflating balloons and airships.
- Cryogenics: Its extremely low boiling point is crucial in cooling superconducting magnets and other low-temperature applications.
- Leak Detection: Its low molecular weight allows it to detect leaks in high-vacuum systems.
- Welding and Shielding: Helium is used as a shielding gas in welding to prevent oxidation.
Lithium (Li): The Lightest Alkali Metal
Lithium, the third element, is the lightest alkali metal, characterized by its high reactivity.
Properties of Lithium:
- Reactive: It readily reacts with water and oxygen, exhibiting typical alkali metal characteristics.
- Low Density: It has a relatively low density compared to other metals.
- Conductivity: Lithium is a good conductor of electricity and heat.
- Silver-White in Appearance: In its pure form, lithium displays a shiny, silvery-white appearance.
Applications of Lithium:
- Batteries: Lithium-ion batteries, ubiquitous in portable electronics and electric vehicles, rely on lithium's electrochemical properties.
- Lubricants: Lithium-based greases are used as high-temperature lubricants.
- Ceramics and Glass: Lithium compounds are used in the production of specialized ceramics and glasses.
- Psychiatric Medications: Lithium salts are used in the treatment of bipolar disorder.
Beryllium (Be): A Lightweight, Strong Metal
Beryllium, the fourth element, is a lightweight yet surprisingly strong alkaline earth metal.
Properties of Beryllium:
- Lightweight and Strong: It boasts a high strength-to-weight ratio, making it attractive for aerospace applications.
- High Thermal Conductivity: Beryllium efficiently conducts heat.
- Toxic: Beryllium and its compounds are highly toxic, requiring careful handling.
- Greyish-White Metal: Beryllium appears as a steel-grey metal.
Applications of Beryllium:
- Aerospace: Used in high-performance aircraft components due to its strength and lightweight nature.
- Nuclear Reactors: Beryllium is used as a neutron reflector in some nuclear reactors.
- X-ray Windows: Its transparency to X-rays makes it useful in X-ray equipment.
- Electronics: Beryllium is found in some specialized electronic components.
Boron (B): A Metalloid with Versatile Uses
Boron, the fifth element, is a metalloid, exhibiting properties of both metals and nonmetals.
Properties of Boron:
- Metalloid: It displays properties intermediate between metals and nonmetals.
- High Melting Point: Boron has a very high melting point.
- Hard and Brittle: It's a hard but brittle material.
- Semiconductor Properties: Some boron forms exhibit semiconductor properties.
Applications of Boron:
- Glass: Boron compounds are crucial ingredients in borosilicate glass (e.g., Pyrex).
- Semiconductors: Boron is a crucial dopant in semiconductor technology.
- Detergents: Boron compounds are used in certain detergents.
- Nuclear Applications: Boron compounds are used in nuclear control rods.
Carbon (C): The Basis of Life
Carbon, the sixth element, is the cornerstone of organic chemistry and the foundation of life as we know it.
Properties of Carbon:
- Allotropes: Carbon exists in various allotropic forms, including diamond, graphite, and fullerenes, each with distinct properties.
- Tetravalent: Carbon atoms can form four covalent bonds, leading to a vast array of organic molecules.
- Essential for Life: Carbon is the building block of all living organisms.
- Abundant: Carbon is found extensively in the environment, both organically and inorganically.
Applications of Carbon:
- Organic Chemistry: The basis of all organic compounds and thus countless applications.
- Diamonds: Used in jewelry and industrial cutting tools.
- Graphite: Used in pencils, lubricants, and electrodes.
- Fullerenes and Nanotubes: Explore new frontiers in materials science and nanotechnology.
Nitrogen (N): A Crucial Component of the Atmosphere
Nitrogen, the seventh element, constitutes the majority of Earth's atmosphere.
Properties of Nitrogen:
- Gaseous State: Nitrogen exists as a diatomic gas (N₂) under standard conditions.
- Inert: Relatively inert at room temperature.
- Essential Nutrient: Crucial for plant growth and protein synthesis.
- Colorless and Odorless: Nitrogen gas is colorless and odorless.
Applications of Nitrogen:
- Fertilizers: Used in the production of ammonia-based fertilizers.
- Food Packaging: Used to preserve food and extend shelf life.
- Cryogenics: Liquid nitrogen is used as a refrigerant.
- Chemical Industry: Used in the synthesis of various nitrogen-containing compounds.
Oxygen (O): Essential for Respiration
Oxygen, the eighth element, is vital for respiration and many combustion processes.
Properties of Oxygen:
- Gaseous State: Exists as a diatomic gas (O₂) under standard conditions.
- Highly Reactive: Readily reacts with many substances, supporting combustion and respiration.
- Colorless and Odorless: Pure oxygen gas is colorless and odorless.
- Allotropes: Oxygen has an allotrope, ozone (O₃), with distinct properties.
Applications of Oxygen:
- Respiration: Essential for animal and plant respiration.
- Combustion: Supports burning and various industrial processes.
- Medicine: Used in medical applications, particularly in hospitals.
- Welding: Used as an oxidizer in welding processes.
Fluorine (F): The Most Reactive Halogen
Fluorine, the ninth element, is the most reactive halogen, exhibiting exceptional reactivity.
Properties of Fluorine:
- Highly Reactive: The most electronegative element, readily forming strong bonds.
- Pale Yellow Gas: Exists as a pale yellow gas under standard conditions.
- Toxic: Fluorine and its compounds are highly toxic.
- Powerful Oxidizing Agent: Fluorine is a potent oxidizing agent.
Applications of Fluorine:
- Teflon (Polytetrafluoroethylene): Used in non-stick cookware and other applications.
- Refrigerants: Certain fluorocarbons were used in refrigerants (though many are being phased out).
- Toothpaste: Fluoride compounds are added to toothpaste to strengthen tooth enamel.
- Uranium Enrichment: Used in the enrichment of uranium for nuclear fuel.
Neon (Ne): A Noble Gas with Distinctive Glow
Neon, the tenth element, is a noble gas known for its distinctive red-orange glow.
Properties of Neon:
- Inert: Like other noble gases, neon is chemically inert.
- Glows Red-Orange: Excites with an electrical discharge to produce a characteristic red-orange glow.
- Low Boiling Point: Neon has a very low boiling point.
- Colorless and Odorless: Neon gas is colorless and odorless.
Applications of Neon:
- Neon Signs: Its distinctive glow makes it widely used in neon signs.
- Lasers: Neon is used in certain types of lasers.
- Cryogenics: Liquid neon is used in some cryogenic applications.
- High-Voltage Indicators: Neon is employed in high-voltage indicators.
This detailed exploration of the first ten elements provides a fundamental understanding of their properties, applications, and significance. From the simplest element, hydrogen, to the glowing neon, each element plays a vital role in our world, contributing to a diverse range of technologies and impacting our daily lives. Further exploration into the periodic table will reveal even more fascinating elements and the complex relationships that govern their interactions.
Latest Posts
Latest Posts
-
When Jackson Hears That His Neighbors House Has Been Robbed
Mar 19, 2025
-
Which Word Shares A Word Root With Remember
Mar 19, 2025
-
What Event Happened After The Qin Dynasty Collapsed
Mar 19, 2025
-
The Crossover Point Is That Production Quantity Where
Mar 19, 2025
-
Permanent Colors Containing Para Dyes Would Fall Into Which Color Category
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about First Ten Elements On The Period Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
