Heat Is A Measure Of The Random Of Molecules.
Breaking News Today
Mar 14, 2025 · 6 min read
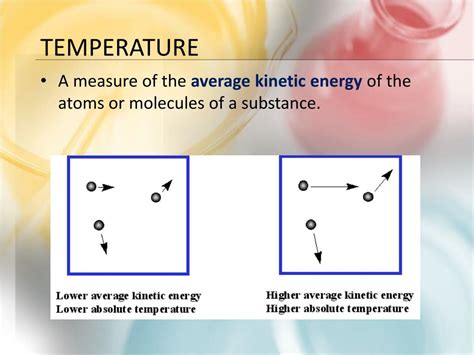
Table of Contents
Heat: A Measure of Molecular Randomness
Heat, a concept we encounter daily, is far more than just a sensation of warmth or coldness. At its core, heat is a manifestation of the random kinetic energy of molecules. Understanding this fundamental relationship is crucial to comprehending various physical phenomena, from the melting of ice to the power of a steam engine. This article delves into the intricate connection between heat and molecular randomness, exploring its implications across different scientific disciplines.
What is Heat? A Microscopic Perspective
While we often perceive heat as a fluid-like substance that flows from hotter objects to colder ones, a deeper understanding requires a microscopic viewpoint. Heat, scientifically, is the transfer of thermal energy. This thermal energy, in turn, is directly tied to the kinetic energy of the molecules that make up a substance.
The molecules within any substance – solid, liquid, or gas – are in constant motion. This motion isn't uniform; it's chaotic and random. Molecules vibrate, rotate, and translate (move from one location to another), constantly colliding with each other and their surroundings. The average kinetic energy of these random molecular motions is directly proportional to the temperature of the substance.
Higher temperature means higher average kinetic energy. The molecules are moving faster, vibrating more vigorously, and colliding with greater force. Conversely, a lower temperature signifies lower average kinetic energy; the molecules are moving slower and less energetically.
Heat, therefore, is the net transfer of this kinetic energy from a region of higher average kinetic energy (higher temperature) to a region of lower average kinetic energy (lower temperature). This transfer continues until thermal equilibrium is reached, where the average kinetic energy of the molecules is the same throughout.
Temperature vs. Heat: Key Differences
It's essential to distinguish between heat and temperature. While closely related, they are distinct concepts:
-
Temperature: A measure of the average kinetic energy of the molecules in a substance. It's an intensive property, meaning it doesn't depend on the amount of substance. A small cup of boiling water and a large pot of boiling water have the same temperature.
-
Heat: The transfer of thermal energy from a hotter object to a colder object. It's an extensive property, meaning it depends on the amount of substance. A large pot of boiling water contains significantly more heat than a small cup of boiling water, even though they have the same temperature.
The Randomness Factor: A Deeper Dive
The "randomness" in the definition of heat is crucial. If all the molecules were moving in a perfectly coordinated, uniform manner, there would be no heat transfer. The randomness implies a distribution of kinetic energies among the molecules. Some molecules will have higher kinetic energies than others; some will be moving faster, others slower. This chaotic distribution is a fundamental characteristic of thermal energy.
This randomness isn't just a theoretical concept; it has profound real-world consequences. The Second Law of Thermodynamics, a cornerstone of physics, states that the total entropy (a measure of disorder or randomness) of an isolated system can only increase over time or remain constant in ideal cases where the system is in a steady state or undergoing a reversible process. The spontaneous flow of heat from hot to cold is a direct consequence of this law. The increase in entropy reflects the increasing randomness of the molecular motions as heat is transferred.
Manifestations of Heat and Molecular Randomness
The connection between heat and molecular randomness manifests itself in many everyday phenomena:
1. Phase Transitions:
The changes of state (solid, liquid, gas) are driven by changes in the average kinetic energy of molecules and hence, heat transfer.
-
Melting: As heat is added to a solid, the average kinetic energy of its molecules increases. Eventually, the molecules gain enough energy to overcome the attractive forces holding them in a fixed lattice structure, resulting in the transition to a liquid.
-
Boiling: Further heating increases the kinetic energy even more. At the boiling point, the molecules have enough energy to escape the liquid phase entirely, becoming a gas.
-
Freezing and Condensation: These are the reverse processes, where heat is removed, causing a decrease in kinetic energy and a phase transition to a more ordered state.
2. Thermal Expansion:
Most substances expand when heated and contract when cooled. This is because the increased kinetic energy of molecules at higher temperatures causes them to move further apart, leading to an increase in volume.
3. Heat Transfer Mechanisms:
Heat transfer occurs through three primary mechanisms:
-
Conduction: The transfer of heat through direct contact between molecules. The more energetic molecules transfer some of their kinetic energy to their less energetic neighbors.
-
Convection: The transfer of heat through the movement of fluids (liquids or gases). Warmer, less dense fluids rise, while cooler, denser fluids sink, creating convection currents.
-
Radiation: The transfer of heat through electromagnetic waves. All objects emit thermal radiation, the intensity of which is related to their temperature.
Heat and Work: Interconversion of Energy
Heat is a form of energy, and like other forms of energy, it can be converted into work. This is the principle behind heat engines, such as internal combustion engines and steam turbines. These engines harness the random kinetic energy of molecules (heat) to perform mechanical work, such as powering a car or generating electricity. The efficiency of such conversion is limited by the Second Law of Thermodynamics.
Applications and Implications
The understanding of heat and molecular randomness has broad applications across various fields:
-
Engineering: Designing efficient heat engines, optimizing thermal management in electronic devices, and developing advanced materials with specific thermal properties.
-
Climate Science: Understanding the greenhouse effect, predicting climate change, and developing strategies for mitigating its impact.
-
Materials Science: Developing new materials with tailored thermal properties, such as high thermal conductivity for heat sinks or low thermal conductivity for insulation.
-
Medicine: Understanding heat transfer in the human body, developing therapeutic applications of heat and cold, and designing medical devices that efficiently manage temperature.
-
Cooking: Applying principles of heat transfer to cook food effectively and safely.
Conclusion
Heat, at its most fundamental level, is a measure of the random kinetic energy of molecules. This seemingly simple concept is the foundation for a vast range of phenomena in physics, chemistry, engineering, and other scientific disciplines. Understanding the relationship between heat and molecular randomness is not only crucial for grasping the workings of the physical world but also for developing innovative technologies and addressing pressing global challenges. The ongoing research in this area continues to unveil new insights and applications, reinforcing the importance of this fundamental scientific principle. From the everyday experience of feeling warmth to the complex processes driving climate change, the random dance of molecules underlies it all.
Latest Posts
Latest Posts
-
The Direct Carry Is Used To Transfer A Patient
Mar 18, 2025
-
The Emancipation Proclamation Of January 1 1863 Quizlet
Mar 18, 2025
-
These Cards Will Get You Drunk Quizlet
Mar 18, 2025
-
Did Quizlet Get Rid Of Q Chat
Mar 18, 2025
-
Myasthenia Gravis Is An Autoimmune Disease In Which Quizlet
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Heat Is A Measure Of The Random Of Molecules. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
