How Many Covalent Bonds Can Carbon Form
Breaking News Today
Mar 25, 2025 · 6 min read
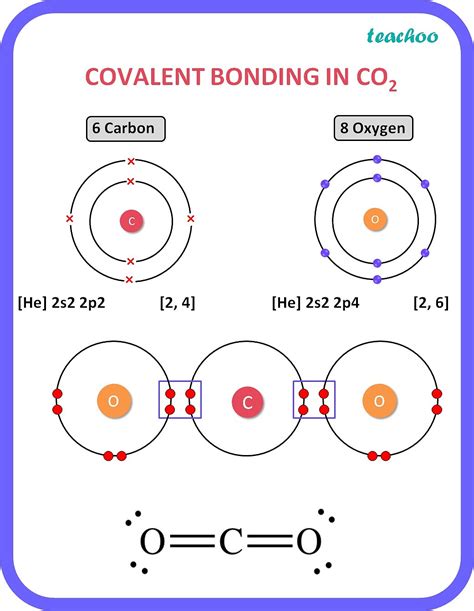
Table of Contents
How Many Covalent Bonds Can Carbon Form? The Remarkable Versatility of Carbon
Carbon, the backbone of life, boasts a unique characteristic that underpins its incredible versatility: its ability to form four covalent bonds. This seemingly simple fact is the foundation for the vast complexity of organic chemistry and the astonishing diversity of molecules found in living organisms and beyond. Understanding why carbon forms four bonds, and the implications of this capability, is crucial to grasping the fundamentals of chemistry and biology.
The Electronic Structure: The Key to Carbon's Bonding Capacity
The answer lies in carbon's electronic structure. Carbon possesses six electrons: two in its innermost shell (the 1s orbital) and four in its outermost shell (the 2s and 2p orbitals). These four electrons in the outer shell are its valence electrons, meaning they participate in chemical bonding. To achieve a stable, low-energy configuration, carbon strives to fill its outermost electron shell, ideally achieving an octet (eight electrons). It can accomplish this by forming four covalent bonds, sharing one electron with each of four other atoms.
Orbital Hybridization: A Deeper Dive into Bonding
While a simplistic view might suggest carbon forms four bonds using one electron from each of its four 2s and 2p orbitals, the reality is more nuanced. Experimental evidence indicates that carbon's bonds are not simply s and p bonds, but rather a combination, a process known as orbital hybridization. The four valence electrons rearrange themselves into four equivalent hybrid orbitals, called sp³ orbitals. This hybridization allows for the formation of four strong, equivalent sigma (σ) bonds, pointing towards the corners of a tetrahedron—a three-dimensional geometric shape.
This sp³ hybridization is responsible for the tetrahedral geometry frequently observed in organic molecules like methane (CH₄), where each hydrogen atom shares a single electron with one of carbon's sp³ orbitals, forming four strong C-H bonds. The bond angles in methane are approximately 109.5°, consistent with a tetrahedral arrangement.
Beyond sp³: Exploring Other Hybridization States
While sp³ hybridization is the most common, carbon exhibits remarkable flexibility in its bonding capabilities, adopting other hybridization states depending on the molecular environment. These include:
sp² Hybridization: Double Bonds and Trigonal Planar Geometry
In molecules containing double bonds, such as ethene (C₂H₄), carbon undergoes sp² hybridization. One of the 2p orbitals remains unhybridized, while the 2s orbital and two of the 2p orbitals hybridize to form three sp² orbitals. These sp² orbitals arrange themselves in a trigonal planar geometry with bond angles of approximately 120°. The remaining unhybridized 2p orbital overlaps sideways with a similar orbital on the other carbon atom to form a weaker pi (π) bond, in addition to the sigma (σ) bond formed by the overlap of sp² orbitals.
This combination of a sigma bond and a pi bond constitutes the double bond in ethene. The presence of the pi bond restricts rotation around the carbon-carbon double bond, leading to the existence of cis-trans isomers.
sp Hybridization: Triple Bonds and Linear Geometry
In molecules containing triple bonds, such as ethyne (C₂H₂), carbon adopts sp hybridization. Two of the 2p orbitals remain unhybridized, while the 2s orbital and one 2p orbital hybridize to form two sp orbitals. These sp orbitals are arranged linearly with a bond angle of 180°. Two pi (π) bonds are formed by the side-by-side overlap of the remaining two unhybridized 2p orbitals on each carbon atom, in addition to the sigma (σ) bond formed by the overlap of the sp orbitals.
This combination of one sigma bond and two pi bonds constitutes the triple bond in ethyne. The linear geometry and the presence of two pi bonds significantly impact the reactivity and properties of molecules containing triple bonds.
The Implications of Carbon's Tetravalency: A World of Organic Chemistry
The ability of carbon to form four covalent bonds has profound implications, leading to the enormous diversity of organic molecules. This tetravalency enables carbon to:
-
Form long chains: Carbon atoms can bond to each other, forming long chains and branched structures, forming the backbone of many organic molecules. These chains can be straight, branched, or even cyclic.
-
Form rings: Carbon atoms can bond to each other to form stable rings, ranging in size from three to many atoms. The presence of rings significantly influences the shape and properties of the molecule.
-
Form complex structures: The combination of chains and rings allows for the creation of incredibly complex and diverse structures, providing the foundation for the vast array of organic molecules found in nature.
-
Form diverse functional groups: Carbon's ability to bond with various other atoms (e.g., hydrogen, oxygen, nitrogen, sulfur, halogens) leads to the formation of diverse functional groups, such as alcohols, ketones, carboxylic acids, amines, and others. These functional groups significantly influence the chemical reactivity and properties of the molecule.
Beyond Organic Chemistry: Carbon's Role in Inorganic Materials
While carbon's role in organic chemistry is undeniable, its tetravalency also plays a crucial role in various inorganic materials. Consider:
-
Diamond: In diamond, each carbon atom forms four strong sp³ hybridized bonds with four other carbon atoms in a giant covalent structure. This creates a rigid, three-dimensional network, resulting in diamond's exceptional hardness and high refractive index.
-
Graphite: In graphite, carbon atoms are arranged in layers of hexagonal rings. Each carbon atom forms three sp² hybridized bonds within its layer, and the remaining p orbital forms a delocalized pi (π) system above and below the layer. The weak van der Waals forces between the layers account for graphite's softness and lubricating properties.
-
Fullerenes: Fullerenes, like buckminsterfullerene (C₆₀), are molecules composed entirely of carbon atoms arranged in a spherical or ellipsoidal shape. Each carbon atom forms three bonds, with sp² hybridization in most cases, creating a hollow structure.
-
Carbon nanotubes: These cylindrical molecules consist of rolled-up sheets of graphite, possessing remarkable strength and electrical conductivity.
Conclusion: A Versatile Atom with Limitless Possibilities
The ability of carbon to form four covalent bonds is the cornerstone of its remarkable versatility. This simple fact underpins the vastness of organic chemistry, enabling the creation of an almost infinite number of molecules with diverse structures and properties. From the simplest organic molecules to the complex biomolecules that make up life, and from the hardest material known to man to the pliable graphite used in pencils, carbon's tetravalency dictates the world around us. Further exploration into the intricacies of carbon's bonding continues to unveil new and exciting possibilities, constantly expanding our understanding of this extraordinary element. The study of carbon and its bonding remains a vibrant and ever-evolving field, holding the key to countless advancements in materials science, medicine, and technology.
Latest Posts
Latest Posts
-
Olga Lucia No Encuentra Su Cepillo Azul
Mar 28, 2025
-
What Was Napoleon Able To Accomplish During Peacetime
Mar 28, 2025
-
What Insect Symbolizes Both Death And Rebirth
Mar 28, 2025
-
Prevention Of The Spread Of Infections Begins And Ends With
Mar 28, 2025
-
The Entry To Establish A Petty Cash Fund Includes
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about How Many Covalent Bonds Can Carbon Form . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
