Match The Type Of Bond With Its Description.
Breaking News Today
Mar 30, 2025 · 6 min read
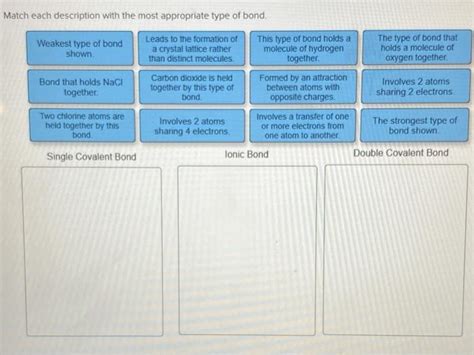
Table of Contents
Match the Type of Bond with its Description: A Comprehensive Guide
Understanding chemical bonds is fundamental to comprehending the behavior of matter. From the simplest molecules to the most complex biological systems, the types of bonds holding atoms together dictate their properties and interactions. This comprehensive guide will delve into the various types of chemical bonds, matching each with its accurate description, clarifying misconceptions, and providing examples to solidify your understanding.
Covalent Bonds: Sharing is Caring
Covalent bonds are formed when two atoms share one or more pairs of electrons. This sharing occurs because both atoms achieve a more stable electron configuration, typically resembling a noble gas (full outer electron shell). The strength of a covalent bond is determined by the number of shared electron pairs and the electronegativity difference between the atoms involved.
Types of Covalent Bonds:
- Single Covalent Bond: One pair of electrons is shared between two atoms. Example: The bond in a hydrogen molecule (H₂).
- Double Covalent Bond: Two pairs of electrons are shared between two atoms. Example: The carbon-oxygen bond in carbon dioxide (CO₂).
- Triple Covalent Bond: Three pairs of electrons are shared between two atoms. Example: The nitrogen-nitrogen bond in nitrogen gas (N₂).
- Polar Covalent Bond: This occurs when the atoms sharing electrons have a significant difference in electronegativity. This results in an unequal sharing of electrons, creating a polar molecule with a slightly positive end and a slightly negative end. Example: The oxygen-hydrogen bond in water (H₂O). Oxygen is more electronegative than hydrogen, pulling the shared electrons closer to itself.
- Nonpolar Covalent Bond: This type of bond forms when the atoms involved have similar or identical electronegativities. Electrons are shared equally, resulting in a nonpolar molecule. Example: The bond in a chlorine molecule (Cl₂).
Characteristics of Covalent Compounds:
- Generally have low melting and boiling points. This is because the intermolecular forces (forces between molecules) are relatively weak.
- Many are gases or liquids at room temperature.
- Poor conductors of electricity and heat. This is because they do not contain free-moving charged particles (ions or electrons).
- Often soluble in nonpolar solvents but not in polar solvents like water.
Ionic Bonds: Opposites Attract
Ionic bonds are formed through the electrostatic attraction between oppositely charged ions. This occurs when one atom (usually a metal) donates one or more electrons to another atom (usually a nonmetal), forming a cation (positively charged ion) and an anion (negatively charged ion). The strong electrostatic force between these ions creates a stable ionic bond.
Formation of Ionic Bonds:
The formation of an ionic bond involves the transfer of electrons from a less electronegative atom (metal) to a more electronegative atom (nonmetal). The metal atom loses electrons to achieve a stable electron configuration, becoming a cation. The nonmetal atom gains electrons, achieving a stable configuration and becoming an anion.
Characteristics of Ionic Compounds:
- Generally have high melting and boiling points. The strong electrostatic forces between ions require significant energy to overcome.
- Usually solids at room temperature.
- Often crystalline structures. The regular arrangement of ions maximizes electrostatic attraction.
- Good conductors of electricity when molten or dissolved in water. This is because the ions are free to move and carry charge.
- Often soluble in polar solvents like water, due to the interaction between the ions and the polar water molecules.
Metallic Bonds: A Sea of Electrons
Metallic bonds are found in metals and are responsible for their unique properties. In metallic bonding, valence electrons are delocalized, meaning they are not associated with any particular atom but rather move freely throughout the metal lattice. This creates a "sea" of electrons surrounding positively charged metal ions.
Characteristics of Metallic Compounds:
- Excellent conductors of heat and electricity. The free-moving electrons can easily transfer energy and charge.
- Malleable and ductile. The "sea" of electrons allows the metal ions to slide past each other without disrupting the bond.
- Lustrous (shiny). The free electrons interact with light, giving metals their characteristic shine.
- High melting and boiling points (generally, but varies greatly depending on the metal). The strong metallic bonds require considerable energy to break.
Hydrogen Bonds: A Special Case
Hydrogen bonds are a type of intermolecular force, not a true chemical bond. They are relatively weak compared to covalent or ionic bonds. They occur between a hydrogen atom bonded to a highly electronegative atom (like oxygen, nitrogen, or fluorine) and another electronegative atom in a different molecule. The electronegative atom pulls the electron density away from the hydrogen atom, creating a partial positive charge (δ+) on the hydrogen and a partial negative charge (δ-) on the electronegative atom. This allows for a relatively strong electrostatic attraction between the partially positive hydrogen and the partially negative atom in another molecule.
Importance of Hydrogen Bonds:
Hydrogen bonds are crucial for many biological processes. They are responsible for:
- The high boiling point of water.
- The structure of proteins and DNA.
- The properties of many biological molecules.
Van der Waals Forces: Weak but Widespread
Van der Waals forces are weak intermolecular forces that arise from temporary fluctuations in electron distribution around atoms and molecules. These forces are present in all molecules, but they are especially significant in nonpolar molecules where other intermolecular forces are absent.
Types of Van der Waals Forces:
- London Dispersion Forces: These are the weakest type of Van der Waals force and are present in all molecules. They arise from temporary dipoles created by the random movement of electrons.
- Dipole-Dipole Forces: These forces occur between polar molecules and are stronger than London dispersion forces. They arise from the attraction between the partially positive end of one molecule and the partially negative end of another.
Matching Bond Types with Descriptions: A Summary Table
| Bond Type | Description | Example | Strength | Properties |
|---|---|---|---|---|
| Covalent | Atoms share electrons. | H₂, H₂O, CO₂ | Variable | Low melting/boiling points (generally), poor conductors |
| Ionic | Electrostatic attraction between oppositely charged ions formed by electron transfer. | NaCl, MgCl₂ | Strong | High melting/boiling points, good conductors (molten or dissolved), often crystalline |
| Metallic | Delocalized electrons in a sea of electrons surrounding metal cations. | Cu, Fe, Al | Strong | Excellent conductors, malleable, ductile, lustrous |
| Hydrogen Bond | Attraction between a hydrogen atom bonded to a highly electronegative atom and another electronegative atom. | Water (H₂O) molecules | Weak (intermolecular) | Influences boiling point, crucial for biological structures |
| Van der Waals | Weak intermolecular forces arising from temporary fluctuations in electron distribution. | Noble gases, nonpolar hydrocarbons | Weak (intermolecular) | Affects physical properties like boiling point and solubility, especially important in nonpolar substances |
This table provides a concise summary of the different types of bonds, their characteristics, and examples. Understanding these distinctions is vital for comprehending the properties and behaviors of various substances in chemistry and beyond. Remember that the strength of a bond is crucial in determining the properties of a substance, and the interplay between different types of bonds can lead to complex and fascinating material behavior. Further study into the nuances of each bond type and their applications will enhance your understanding of the fundamental principles of chemistry.
Latest Posts
Latest Posts
-
From A Security Perspective The Best Rooms Are Directly
Apr 01, 2025
-
Software Lab Simulation 21 1 Linux File System
Apr 01, 2025
-
Which Of The Following Drugs Is Not A Sedative Hypnotic
Apr 01, 2025
-
Advanced Hardware Lab 1 1 Identify Internal Parts Of A Computer
Apr 01, 2025
-
The Process For Obtaining Qualified People Is
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Match The Type Of Bond With Its Description. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
