Most Of The Volume Of An Atom Is Occupied By
Breaking News Today
Mar 16, 2025 · 5 min read
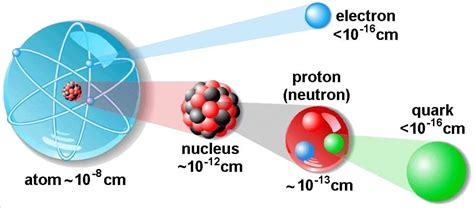
Table of Contents
Most of the Volume of an Atom is Occupied by… Empty Space?
The seemingly simple question, "Most of the volume of an atom is occupied by what?" leads us down a fascinating rabbit hole into the quantum realm. The intuitive answer – a solid, dense mass – is dramatically incorrect. The truth is far stranger and more profound. The vast majority of an atom's volume is, in fact, empty space. This seemingly paradoxical statement is key to understanding the nature of matter itself.
Delving into the Atomic Structure: A Subatomic Safari
To grasp this concept, we must first journey into the heart of the atom. We're talking about a scale so minuscule that billions fit on the head of a pin! The atom isn't a solid, indivisible particle as ancient philosophers believed. Instead, it's a complex system composed of even smaller constituents:
1. The Nucleus: The Dense Core
At the atom's center resides the nucleus, a tiny, incredibly dense region containing two types of particles:
- Protons: Positively charged particles with a mass approximately 1836 times greater than that of an electron. The number of protons determines the element – for example, hydrogen has one proton, helium has two, and so on.
- Neutrons: Neutrally charged particles with a mass slightly larger than that of a proton. Neutrons play a crucial role in nuclear stability. Isotopes of an element differ in the number of neutrons.
The nucleus accounts for almost all of the atom's mass, concentrated in this incredibly small region.
2. The Electron Cloud: A Realm of Probability
Surrounding the nucleus is the electron cloud, a region where electrons orbit the nucleus. Unlike the planets orbiting the sun in our solar system, electrons don't follow precise, well-defined paths. Their positions are governed by the principles of quantum mechanics. Instead of orbits, we speak of orbitals, regions of space where there's a high probability of finding an electron.
Electrons are negatively charged particles with an incredibly small mass, significantly less than that of a proton or neutron. They are responsible for the chemical properties of an atom and its interactions with other atoms.
The Vast Emptiness: Why Atoms Aren't Solid
The crucial point here is the sheer size difference between the nucleus and the electron cloud. Imagine the nucleus as a tiny marble at the center of a football stadium. The electrons would be like a few gnats buzzing around that stadium. The rest of the stadium—the overwhelming majority of the space—would be empty.
This empty space is not truly “nothing.” It’s governed by the laws of quantum mechanics, where things aren't so easily defined in terms of classical physics. There are probabilistic distributions of electrons within orbitals, and the forces governing the interactions between these particles dictate the atom’s properties.
The vast majority of an atom’s volume is occupied by this seemingly empty space, where the influence of the nucleus and the electrons defines the atom’s structure and interactions.
The Implications of Atomic Emptiness: A World of Consequences
The "emptiness" of the atom has profound implications for our understanding of the world:
1. The Nature of Matter: From Solid to Mostly Void
Our perception of solidity is an illusion at the atomic level. What we perceive as solid objects are actually vast collections of atoms, mostly empty space, held together by electromagnetic forces. These forces arise from the interactions between the charged particles within the atoms.
2. Chemical Bonding and Interactions: Dancing in the Void
The interactions between atoms, which govern chemical reactions and the formation of molecules, are a result of the interplay of these forces between electron clouds. Atoms "touch" each other only when their electron clouds overlap, and it is this overlap that leads to bonds.
3. The Importance of Quantum Mechanics: Beyond Classical Physics
The bizarre nature of the atom highlights the limitations of classical physics in describing the microscopic world. Quantum mechanics is crucial in understanding the behavior of subatomic particles and their interactions, explaining the probabilistic nature of electron locations and the wave-particle duality of matter.
4. Technological Advancements: Harnessing the Quantum Realm
The understanding of atomic structure and quantum mechanics has led to incredible technological advancements, including transistors, lasers, and advanced imaging techniques. Further research into quantum mechanics promises to revolutionize fields like computing, materials science, and medicine.
Analogies to Illustrate the Vast Emptiness
Several analogies help visualize the relative scale:
- The marble and the stadium: As mentioned before, this classic analogy helps understand the vast difference in scale between the nucleus and the atom's overall size.
- The solar system: While not a perfect analogy due to the differences between gravitational and electromagnetic forces, it helps visualize the central nucleus and the orbiting electrons (though the electrons' behavior is far more complex).
- A fly in a cathedral: A fly buzzing around a massive cathedral represents the electron’s relative size compared to the overall volume of the atom.
Beyond the Atom: Exploring the Subatomic World
The subatomic world holds even more mysteries. Protons and neutrons themselves are composed of even smaller particles called quarks, held together by the strong nuclear force. Understanding the intricacies of these interactions is a crucial area of ongoing research in particle physics.
Conclusion: Embracing the Quantum Enigma
The answer to "Most of the volume of an atom is occupied by what?" is, surprisingly, mostly empty space. This seemingly paradoxical answer underlines the incredible complexity and counter-intuitive nature of the quantum world. Our macroscopic experience of solidity is a deceptive illusion, a reflection of the forces and interactions of these tiny particles within the atom's vast, mostly empty volume.
Understanding this fundamental truth provides a deeper appreciation for the nature of matter, the power of quantum mechanics, and the extraordinary technological potential hidden within the seemingly empty spaces of atoms. As we continue to probe deeper into the subatomic world, we’ll undoubtedly uncover further mysteries and continue to refine our understanding of the cosmos, starting with the fundamental building blocks of everything: atoms. The journey into this miniature universe promises to yield even more remarkable discoveries in the future.
Latest Posts
Latest Posts
-
Unit 5 Progress Check Mcq Ap Bio
Mar 16, 2025
-
Which Macronutrient Is Vital For Every Function Of The Body
Mar 16, 2025
-
Which Is The Safest To Make A Two Point Turn
Mar 16, 2025
-
When Using A Transfer Belt The Na Should
Mar 16, 2025
-
For A Child Cpr Involving A Covid 19 Positive Victim
Mar 16, 2025
Related Post
Thank you for visiting our website which covers about Most Of The Volume Of An Atom Is Occupied By . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
