Question Usher You Are Given An Alkene
Breaking News Today
Mar 24, 2025 · 5 min read
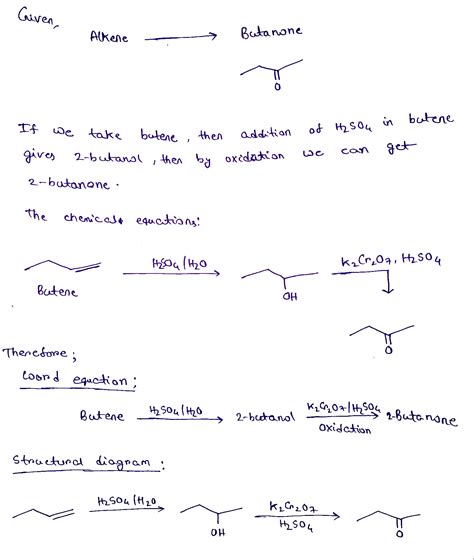
Table of Contents
An Alkene Presents: A Comprehensive Guide to Reactions and Mechanisms
Ushered into the world of organic chemistry, we find ourselves face-to-face with alkenes – unsaturated hydrocarbons boasting a carbon-carbon double bond. This seemingly simple structural feature is the source of a wealth of fascinating reactivity. This article will delve deep into the multifaceted world of alkene reactions, exploring various mechanisms and their applications. We'll cover everything from electrophilic addition to oxidation and polymerization, providing a comprehensive understanding for both students and seasoned chemists.
Understanding the Carbon-Carbon Double Bond: The Heart of Alkene Reactivity
The defining characteristic of alkenes is the carbon-carbon double bond. This bond consists of a strong sigma (σ) bond and a weaker pi (π) bond. The π bond, formed by the sideways overlap of p-orbitals, is crucial to alkene reactivity. Its relatively loose electron density makes it susceptible to attack by electrophiles, setting the stage for a variety of addition reactions.
Key Characteristics Influencing Reactivity:
- Electron Density: The electron-rich nature of the π bond makes alkenes nucleophilic, meaning they readily react with electron-deficient species (electrophiles).
- Steric Hindrance: The size and arrangement of substituents around the double bond can significantly influence the rate and selectivity of reactions. Bulky groups can hinder the approach of reactants.
- Substitution Pattern: The number of alkyl groups attached to the double-bonded carbons (mono-, di-, tri-, tetra-substituted) affects the electron density and steric hindrance, thereby impacting reactivity.
Electrophilic Addition: The Cornerstone of Alkene Chemistry
Electrophilic addition is the most prevalent reaction type for alkenes. It involves a two-step mechanism where an electrophile (electron-loving species) attacks the π bond, forming a carbocation intermediate. This intermediate is then attacked by a nucleophile (electron-rich species), leading to the formation of a saturated product.
Markovnikov's Rule: Predicting the Product
In the addition of unsymmetrical reagents (e.g., HBr, HCl), Markovnikov's rule provides a crucial guideline for predicting the major product. This rule states that the electrophile (usually the proton) adds to the carbon atom with the fewer number of alkyl substituents, leading to the more substituted carbocation intermediate, which is more stable.
Examples of Electrophilic Addition Reactions:
-
Hydrohalogenation (HX): The addition of hydrogen halides (HCl, HBr, HI) to alkenes, resulting in haloalkanes. The regioselectivity follows Markovnikov's rule.
-
Hydration (H₂O): The addition of water to alkenes in the presence of an acid catalyst (e.g., H₂SO₄), yielding alcohols. Again, Markovnikov's rule dictates the regioselectivity.
-
Halogenation (X₂): The addition of halogens (Cl₂, Br₂) to alkenes, resulting in vicinal dihalides. This reaction proceeds via a cyclic bromonium or chloronium ion intermediate.
-
Hydrohalogenation with Peroxides (Anti-Markovnikov Addition): The presence of peroxides (ROOR) can reverse the regioselectivity of hydrohalogenation, leading to anti-Markovnikov addition (the halide adds to the less substituted carbon). This occurs through a radical mechanism, rather than an ionic one.
Other Important Alkene Reactions:
Beyond electrophilic addition, alkenes participate in a diverse array of reactions. Let's explore some key examples:
Oxidation Reactions:
Alkenes can be oxidized to various products depending on the oxidizing agent and reaction conditions.
-
Epoxidation: Using peroxyacids (e.g., mCPBA), alkenes can be converted into epoxides (three-membered cyclic ethers).
-
Ozonolysis: Ozone (O₃) cleaves the double bond, forming ozonides, which are then reductively cleaved (e.g., with Zn/acetic acid) to yield aldehydes or ketones.
-
Hydroxylation: Using osmium tetroxide (OsO₄) or potassium permanganate (KMnO₄), alkenes can be converted into vicinal diols (1,2-diols).
Reduction Reactions:
Alkenes can be reduced (addition of hydrogen) using catalytic hydrogenation. This reaction requires a metal catalyst (e.g., Pt, Pd, Ni) and hydrogen gas (H₂). The product is an alkane.
Polymerization:
Alkenes are the building blocks for many important polymers. Addition polymerization involves the sequential addition of alkene monomers to form long chains. Examples include polyethylene (from ethene) and polypropylene (from propene).
Allylic Reactions:
Reactions involving the carbon atom adjacent to the double bond (allylic position) are significant. Allylic bromination using N-bromosuccinimide (NBS) is a common example.
Stereochemistry in Alkene Reactions:
The stereochemistry of alkene reactions is crucial. Addition reactions can proceed with either syn (addition from the same side) or anti (addition from opposite sides) stereochemistry. The stereochemical outcome depends on the reaction mechanism and the nature of the intermediate.
Applications of Alkene Reactions:
The reactions of alkenes are fundamental in numerous applications across various fields:
-
Polymer Industry: The production of plastics, rubbers, and fibers relies heavily on alkene polymerization.
-
Pharmaceutical Industry: Many pharmaceuticals contain alkene functional groups, and their reactions are vital in drug synthesis.
-
Petrochemical Industry: Alkenes are key intermediates in the production of fuels and other petrochemicals.
-
Organic Synthesis: Alkenes serve as versatile building blocks in the synthesis of complex organic molecules.
Conclusion:
Alkenes, despite their seemingly simple structure, exhibit a rich and diverse array of reactivity. Understanding the mechanisms and stereochemistry of alkene reactions is essential for anyone working in the field of organic chemistry. This article has provided a comprehensive overview of the major reaction types, their mechanisms, and their significant applications across various industries. Further exploration of specific reactions and their intricacies can lead to a deeper appreciation of the fascinating world of alkene chemistry. From the simple addition of a hydrogen halide to the complex polymerization process, the reactivity of alkenes underlines their pivotal role in organic synthesis and industrial applications. The principles discussed here form a solid foundation for continued learning and exploration in this vital area of chemistry. Remember that constant practice and a thorough understanding of underlying mechanisms are key to mastering this fascinating aspect of organic chemistry.
Latest Posts
Latest Posts
-
When Preparing The Bank Deposit Currency Is
Mar 26, 2025
-
You Are Coupling A Tractor To A Semi Trailer
Mar 26, 2025
-
Which Of These Is A Nonsteroid Hormone
Mar 26, 2025
-
When You Evaluate An Online Document For Sponsorship You Should
Mar 26, 2025
-
User Is Unable To Reach Google Com By Typing The Url
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Question Usher You Are Given An Alkene . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
