Reaction Between A Metal And A Nonmetal Synthesis Or Decomposition
Breaking News Today
Mar 25, 2025 · 7 min read
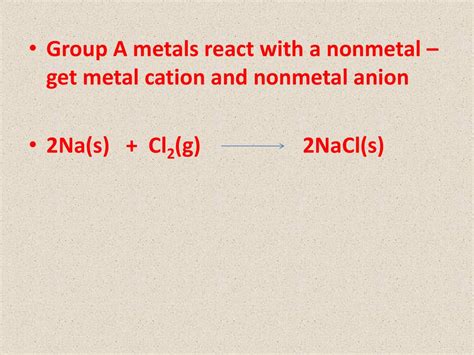
Table of Contents
The Fascinating Dance of Metals and Nonmetals: Synthesis and Decomposition Reactions
The world around us is a testament to the incredible diversity of chemical reactions. Among these, the reactions between metals and nonmetals stand out for their prevalence and importance, forming the backbone of countless materials we encounter daily. These interactions typically fall into two broad categories: synthesis reactions, where a metal and nonmetal combine to form a compound, and decomposition reactions, the reverse process where a compound breaks down into its constituent metal and nonmetal. Understanding these reactions is fundamental to comprehending the behavior of matter and the design of new materials. This comprehensive article will delve deep into the mechanisms, driving forces, and applications of these fascinating chemical interactions.
Synthesis Reactions: Building Compounds from Metals and Nonmetals
Synthesis reactions, also known as combination reactions, involve the direct combination of two or more reactants to form a single product. In the context of metals and nonmetals, this typically results in the formation of an ionic compound. The reaction is driven by the significant difference in electronegativity between the metal (electropositive) and the nonmetal (electronegative). The metal readily loses electrons to achieve a stable electron configuration, while the nonmetal gains these electrons, also achieving stability. This transfer of electrons results in the formation of ions, held together by strong electrostatic forces, creating an ionic bond.
The Mechanism of Synthesis Reactions
The process begins with the interaction between the metal atoms and nonmetal atoms. The metal atoms, having relatively low ionization energies, easily release their valence electrons. These electrons are then readily accepted by the nonmetal atoms, which have high electron affinities. This electron transfer generates positively charged metal cations (M<sup>+</sup>) and negatively charged nonmetal anions (X<sup>-</sup>). The electrostatic attraction between these oppositely charged ions leads to the formation of an ionic lattice, a highly ordered arrangement of cations and anions. The strength of this ionic bond is largely determined by the charge of the ions and the distance between them. Higher charges and smaller ionic radii lead to stronger bonds.
Example: The Reaction Between Sodium (Na) and Chlorine (Cl<sub>2</sub>)
A classic example is the reaction between sodium metal and chlorine gas to form sodium chloride (common table salt):
2Na(s) + Cl<sub>2</sub>(g) → 2NaCl(s)
In this reaction, sodium atoms each lose one electron to become Na<sup>+</sup> ions, while chlorine molecules (Cl<sub>2</sub>) split into individual chlorine atoms, each gaining one electron to become Cl<sup>-</sup> ions. The resulting Na<sup>+</sup> and Cl<sup>-</sup> ions arrange themselves in a regular three-dimensional lattice, forming the crystalline structure of sodium chloride. The energy released during this reaction is substantial, often manifested as heat and light.
Factors Influencing Synthesis Reactions
Several factors can influence the rate and extent of synthesis reactions between metals and nonmetals:
- Reactivity of the metal: More reactive metals, such as alkali metals (Group 1) and alkaline earth metals (Group 2), readily react with nonmetals. Less reactive metals may require higher temperatures or the presence of a catalyst to initiate the reaction.
- Reactivity of the nonmetal: The reactivity of the nonmetal also plays a crucial role. Highly electronegative nonmetals, such as halogens (Group 17), tend to react more vigorously.
- Temperature: Increasing the temperature usually increases the rate of the reaction by providing the necessary activation energy for the reaction to proceed.
- Surface area: A larger surface area of the reactants exposes more atoms to interaction, increasing the rate of reaction. Finely divided metals react faster than bulk metals.
Decomposition Reactions: Breaking Down Compounds
Decomposition reactions are the opposite of synthesis reactions. They involve the breakdown of a single compound into two or more simpler substances. In the context of metal-nonmetal compounds, this means the ionic compound breaks down into its constituent metal and nonmetal. These reactions typically require energy input, such as heat, electricity, or light, to overcome the strong electrostatic forces holding the ionic lattice together.
The Mechanism of Decomposition Reactions
The process of decomposition begins with the absorption of energy. This energy weakens the ionic bonds within the crystal lattice, allowing the ions to become more mobile. Once sufficient energy is provided, the ionic bonds break, releasing the metal cations and nonmetal anions. These ions may then recombine to form simpler compounds or exist as free elements.
Example: The Decomposition of Metal Carbonates
Many metal carbonates decompose upon heating, yielding the metal oxide and carbon dioxide gas. For example, the decomposition of calcium carbonate (limestone):
CaCO<sub>3</sub>(s) → CaO(s) + CO<sub>2</sub>(g)
In this reaction, heat provides the energy to break the bonds within the calcium carbonate lattice. The calcium and oxygen ions remain bonded together to form calcium oxide, while the carbon and oxygen atoms combine to form carbon dioxide gas.
Factors Influencing Decomposition Reactions
Several factors can affect the ease and conditions under which decomposition reactions occur:
- Strength of the ionic bond: Compounds with weaker ionic bonds are easier to decompose than those with stronger bonds. This is often related to the charges of the ions and their sizes.
- Temperature: Higher temperatures generally favor decomposition reactions by providing the necessary energy to break the bonds. The required temperature varies significantly depending on the compound's stability.
- Presence of a catalyst: Certain catalysts can lower the activation energy required for decomposition, making the reaction occur at lower temperatures or at a faster rate.
Applications of Synthesis and Decomposition Reactions
Synthesis and decomposition reactions between metals and nonmetals are crucial in a wide range of applications, including:
- Metallurgy: The extraction of metals from their ores often involves a series of synthesis and decomposition reactions. For example, the reduction of metal oxides using carbon (a nonmetal) is a common method for obtaining pure metals.
- Material Science: Numerous materials with specific properties are synthesized by combining metals and nonmetals. Examples include ceramics, semiconductors, and various alloys.
- Chemical Industry: Many industrial processes rely on synthesis and decomposition reactions involving metals and nonmetals. These include the production of fertilizers, chemicals for various applications, and many more.
- Environmental Science: Understanding these reactions is crucial for environmental remediation. For instance, understanding the decomposition of metal compounds in soil or water helps us manage pollution effectively.
- Energy Production: Some metal-nonmetal compounds play vital roles in energy storage and conversion technologies, such as batteries and fuel cells.
Beyond the Basics: Exploring Complexities
While the fundamental principles outlined above provide a solid understanding of metal-nonmetal reactions, the reality is often more nuanced. Many reactions involve multiple steps, intermediate compounds, and side reactions. For example, the reaction between a metal and an oxidizing acid isn't simply a direct transfer of electrons but a more complex process involving proton transfer and the formation of intermediate species. Similarly, the decomposition of certain compounds can proceed through multiple pathways depending on factors like temperature and pressure.
The Role of Thermodynamics and Kinetics
Thermodynamics determines whether a reaction is feasible, based on the change in Gibbs free energy (ΔG). A negative ΔG indicates a spontaneous reaction, while a positive ΔG indicates a non-spontaneous reaction. However, thermodynamics doesn't tell us how fast the reaction will occur. This is where kinetics comes in. Kinetics studies the rate of a reaction and the factors that influence it, such as activation energy and reaction mechanisms. A reaction may be thermodynamically favorable but kinetically slow, meaning it may require a catalyst or high temperatures to proceed at a reasonable rate.
Exploring Different Nonmetals
The behavior of metals in reactions with different nonmetals can vary significantly. For instance, reactions with halogens (Group 17) are often highly exothermic and produce stable ionic compounds. Reactions with oxygen (Group 16) can result in the formation of metal oxides, which can exhibit a wide range of properties depending on the metal and the oxidation state. Reactions with other nonmetals, like sulfur, phosphorus, and nitrogen, lead to a diverse array of compounds with varied structures and properties.
Conclusion: A Continuous Area of Discovery
The interactions between metals and nonmetals are fundamental to chemistry and have profound implications across various scientific disciplines and technological applications. The synthesis and decomposition reactions discussed in this article represent just a fraction of the rich complexity involved in metal-nonmetal chemistry. Continued research into these reactions will undoubtedly lead to new insights and innovations, paving the way for the development of novel materials and technologies. From the everyday salt we use to advanced materials used in electronics and energy production, the dance of metals and nonmetals continues to shape our world. Understanding the principles governing these reactions is therefore essential for progress in numerous fields.
Latest Posts
Latest Posts
-
Relias Progressive Care Rn Assessment A Answers
Mar 27, 2025
-
How Did He Actually Slow Scientific Progress In Western Europe
Mar 27, 2025
-
Ati Rn Maternal Newborn Online Practice 2023 A
Mar 27, 2025
-
Which Of The Following Is Not A Period Cost
Mar 27, 2025
-
Hay 13 6 Chicas Y 20 12 Chicos
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about Reaction Between A Metal And A Nonmetal Synthesis Or Decomposition . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
