What Are The Five Properties Of A Mineral
Breaking News Today
Mar 17, 2025 · 6 min read
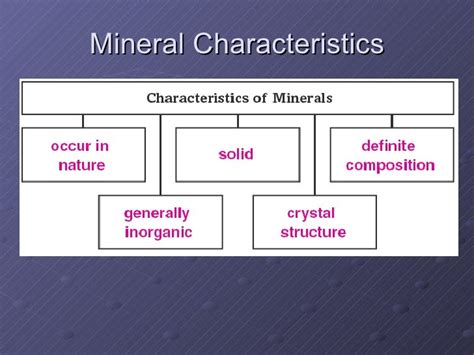
Table of Contents
What are the Five Properties of a Mineral? A Comprehensive Guide
Minerals are the fundamental building blocks of rocks, and understanding their properties is crucial for geologists, mineralogists, and anyone interested in the Earth's composition. While thousands of minerals exist, they all share five key properties: naturally occurring, inorganic, solid, definite chemical composition, and ordered atomic arrangement (crystalline structure). Let's delve deeper into each property.
1. Naturally Occurring
This property might seem straightforward, but it's crucial for distinguishing minerals from synthetic materials. A mineral must be formed by natural geological processes, excluding any human intervention. This means that substances created in a laboratory, even if they possess the other four mineral properties, are not considered minerals. The formation processes can range from the crystallization of magma or lava (igneous rocks), precipitation from solution (sedimentary rocks), or alteration of pre-existing minerals under high pressure and temperature (metamorphic rocks). Understanding the geological context of a mineral's formation often provides clues about its properties and composition. For instance, minerals formed at high temperatures will often exhibit different properties than those formed at low temperatures.
Examples and Counter-Examples:
- Naturally Occurring: Quartz (SiO₂), formed through various geological processes; Diamond (C), formed under immense pressure in the Earth's mantle.
- Not Naturally Occurring: Cubic zirconia (ZrO₂), a synthetic diamond simulant; synthetic sapphire (Al₂O₃ with trace elements), created in laboratories for jewelry. These materials might have similar chemical compositions and crystal structures to their natural counterparts but lack the "naturally occurring" characteristic.
2. Inorganic
This property distinguishes minerals from organic compounds. Minerals are not produced by living organisms or their remains. While some minerals might contain elements that are components of living things (like carbon), the process of their formation does not involve biological activity. Organic compounds, on the other hand, are primarily composed of carbon and hydrogen and are associated with living systems. The distinction is crucial because the formation mechanisms and chemical bonding involved are fundamentally different. Organic molecules are often more complex and less stable in high-temperature geological environments.
Examples and Counter-Examples:
- Inorganic: Feldspar (various silicate compositions), formed through magmatic crystallization; Halite (NaCl), formed through evaporation of saline waters.
- Not Inorganic (Organic): Coal (primarily carbon), formed from the compression of ancient plant matter; Amber (fossilized tree resin), formed from the polymerization of organic compounds. These materials, while found in geological settings, are fundamentally organic in origin.
3. Solid
This property might seem obvious, but it highlights a fundamental difference between minerals and other substances. A mineral must be a solid at standard temperature and pressure (STP). This means it maintains a definite volume and shape. Liquids and gases, while they may contain the same chemical components as minerals, do not exhibit the ordered atomic arrangement required for mineral classification. This is related to the strength of the chemical bonds holding atoms together, with solids possessing much stronger bonds than liquids or gases.
Examples and Counter-Examples:
- Solid: Calcite (CaCO₃), a crystalline solid; Pyrite (FeS₂), a solid with a metallic luster.
- Not Solid: Water (H₂O), liquid at STP; Mercury (Hg), liquid at STP. These substances may exist in solid forms under different conditions (ice, solid mercury), but not at standard temperature and pressure.
4. Definite Chemical Composition
Minerals are characterized by a specific and consistent chemical formula. While there can be some substitution of elements within the mineral structure (leading to variations in the exact composition), there is a defined ratio of elements. This formula reflects the ordered arrangement of atoms within the mineral's crystal structure. This defined composition is what differentiates one mineral from another. For example, quartz (SiO₂) will always have one silicon atom for every two oxygen atoms, while feldspar can have slight variations in its atomic ratios but maintains a general formula reflecting specific groupings of elements.
Examples and Counter-Examples:
- Definite Chemical Composition: Halite (NaCl), always in a 1:1 ratio of sodium to chlorine; Pyrite (FeS₂), always in a 1:2 ratio of iron to sulfur.
- Not Definite Chemical Composition: Rocks are mixtures of multiple minerals and do not have a fixed chemical composition; Solutions, like seawater, have variable concentrations of dissolved components.
5. Ordered Atomic Arrangement (Crystalline Structure)
This is arguably the most critical property defining a mineral. Minerals have an internal structure characterized by a highly ordered arrangement of atoms, ions, or molecules. This ordered structure repeats periodically in three dimensions, creating a crystal lattice. This lattice determines the mineral's physical properties, including its crystal shape, cleavage, hardness, and other characteristics. While some minerals might appear amorphous (lacking a visible crystalline structure), at the atomic level they still exhibit some degree of order. The degree of order may vary, influencing the macroscopic properties and thus the identification of the mineral. X-ray diffraction is a crucial technique used to determine the crystal structure of minerals.
Examples and Counter-Examples:
- Ordered Atomic Arrangement: Quartz (SiO₂), exhibiting a hexagonal crystal system; Diamond (C), featuring a cubic crystal system.
- Not Ordered Atomic Arrangement: Obsidian (volcanic glass), an amorphous material lacking long-range atomic order; Opal, a hydrated amorphous form of silica. These materials often lack the well-defined crystal faces and cleavage planes characteristic of crystalline minerals.
Understanding Mineral Properties for Identification
The five properties are essential for mineral identification. Several other properties, such as color, streak, luster, hardness, cleavage, fracture, and specific gravity, are used in conjunction with the five defining properties to distinguish between minerals. These secondary properties often reflect the chemical composition and crystal structure of the mineral. For example, the hardness of a mineral is directly related to the strength of the chemical bonds in its crystal lattice. Cleavage, the tendency of a mineral to break along specific planes, is a consequence of its atomic arrangement.
Color, while visually striking, can be deceptive, as impurities can significantly alter the color of a mineral. Streak, the color of the mineral powder, provides a more reliable indicator. Luster describes the way light interacts with the mineral's surface (metallic, vitreous, pearly, etc.). Hardness is measured using the Mohs hardness scale, where 1 is talc and 10 is diamond. Cleavage refers to the tendency of a mineral to break along smooth, planar surfaces, while fracture describes irregular breakage patterns. Specific gravity is the ratio of the mineral's density to the density of water.
Conclusion
The five properties – naturally occurring, inorganic, solid, definite chemical composition, and ordered atomic arrangement – are fundamental to understanding what constitutes a mineral. While seemingly simple individually, their combined significance allows for the classification and identification of the thousands of minerals that make up our planet. Understanding these properties is key to appreciating the geological processes that shape the Earth and the materials that form its crust. Further exploration into mineralogy will reveal the fascinating complexities and diversity of these essential components of our world. By combining the fundamental five properties with secondary properties, a comprehensive understanding of mineral classification and identification becomes attainable. Remember that mastering these properties takes time and practice, and observing minerals firsthand is invaluable to solidifying this knowledge.
Latest Posts
Latest Posts
-
Treatment That Includes A Focus On Personal Strengths And Development
Mar 18, 2025
-
Which Is Not A Form Of Maltreatment
Mar 18, 2025
-
If An Individual Is Heterozygous For A Particular Trait
Mar 18, 2025
-
If You Add More Enzyme The Reaction Will
Mar 18, 2025
-
The Purpose Of A Hazcom Program Is To Ensure That
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about What Are The Five Properties Of A Mineral . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
