What Do Electrons In The Same Shell Have In Common
Breaking News Today
Mar 28, 2025 · 6 min read
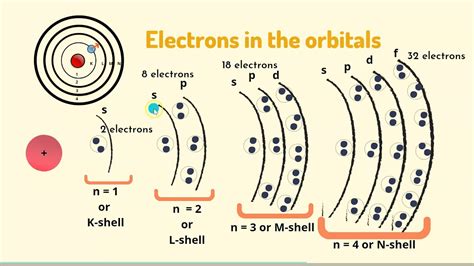
Table of Contents
What Do Electrons in the Same Shell Have in Common? A Deep Dive into Electron Shells and Subshells
Understanding the behavior of electrons within an atom is fundamental to grasping the principles of chemistry and physics. One key concept is the arrangement of electrons into shells, which dictates an atom's properties and reactivity. But what exactly do electrons sharing the same shell have in common? This article delves deep into the characteristics of electron shells, subshells, and orbitals, revealing the similarities and subtle differences within these crucial atomic structures.
The Core Concept: Energy Levels and Electron Shells
Electrons don't orbit the nucleus in a random, chaotic manner. Instead, they occupy specific energy levels, often visualized as concentric shells surrounding the nucleus. These shells, also known as principal energy levels, are designated by the principal quantum number, n, which is a positive integer (1, 2, 3, and so on). The higher the value of n, the higher the energy level and the greater the distance of the shell from the nucleus.
Electrons in the same shell share a crucial similarity: they possess approximately the same energy. This doesn't mean their energies are identical; slight variations exist due to factors we'll explore later. However, the principal quantum number provides a first-order approximation of their energy levels. This shared energy level is the fundamental commonality among electrons within a single shell.
Subshells: A Closer Look Within the Shells
While electrons in the same shell have similar energies, the story doesn't end there. Within each shell (except for the first shell, n = 1), electrons are further organized into subshells. These subshells are defined by the azimuthal quantum number, l, which can take integer values from 0 to n - 1. Each value of l corresponds to a different subshell:
- l = 0: s subshell (spherical shape)
- l = 1: p subshell (dumbbell shape)
- l = 2: d subshell (more complex shapes)
- l = 3: f subshell (even more complex shapes)
Electrons within the same subshell share the same energy level (to a higher degree of accuracy than those in the same shell but different subshells) and the same shape of their atomic orbitals. The slight energy differences between electrons in the same subshell arise from other factors, like electron-electron interactions.
Orbitals: The Three-Dimensional Space Where Electrons Reside
Each subshell contains one or more orbitals. Orbitals are regions of space where there's a high probability of finding an electron. The magnetic quantum number, ml, specifies the orientation of an orbital in space. For example:
- s subshell (l = 0): Has one orbital (ml = 0).
- p subshell (l = 1): Has three orbitals (ml = -1, 0, +1).
- d subshell (l = 2): Has five orbitals (ml = -2, -1, 0, +1, +2).
- f subshell (l = 3): Has seven orbitals (ml = -3, -2, -1, 0, +1, +2, +3).
Electrons within the same orbital have the same energy (to the highest degree of accuracy possible), the same shape, and the same spatial orientation. However, due to the Pauli Exclusion Principle, a single orbital can accommodate a maximum of two electrons, each with opposite spins (represented by the spin quantum number, ms, which can be +1/2 or -1/2).
Similarities Summarized: What Truly Unites Electrons in the Same Shell
To reiterate the key similarities, electrons in the same shell share:
- Similar principal energy levels: They possess approximately the same energy, with the higher shells having higher energy levels.
- The same principal quantum number (n): This number defines the shell and gives a general indication of energy and distance from the nucleus.
- A general proximity to the nucleus: While not all electrons in a shell are equidistant from the nucleus (due to subshells), they are generally located within a similar radial range.
However, it's crucial to remember the nuances: Electrons within the same shell might reside in different subshells, leading to slight variations in their energy levels and orbital shapes. Furthermore, even within the same subshell or orbital, subtle energy differences can occur due to electron-electron interactions.
Differences and Nuances: Understanding the Variations
While the shared characteristics are vital, it's equally important to acknowledge the distinctions:
- Subshell energy differences: Electrons in different subshells within the same shell possess slightly different energy levels. For example, a 2p electron has higher energy than a 2s electron.
- Orbital shapes and orientations: Electrons occupy orbitals with specific shapes (s, p, d, f) and orientations in space. This affects their spatial distribution and interactions.
- Spin: Electrons within the same orbital must have opposite spins, as dictated by the Pauli Exclusion Principle. This subtle difference in spin contributes to the atom's overall magnetic properties.
- Shielding effects: Inner electrons shield outer electrons from the full positive charge of the nucleus. This shielding effect can alter the effective nuclear charge experienced by the outer electrons, leading to slight energy variations.
The Significance of Electron Shell Arrangement
The arrangement of electrons into shells and subshells is paramount for understanding an atom's properties:
- Chemical reactivity: The outermost shell, known as the valence shell, plays a crucial role in determining an atom's reactivity. Atoms tend to react in ways that achieve a stable electron configuration, often by filling their valence shell.
- Spectroscopic properties: Electron transitions between different energy levels (shells and subshells) give rise to the characteristic spectral lines observed in atomic spectroscopy.
- Bonding: The electron configuration influences how atoms form chemical bonds with each other, leading to the formation of molecules and compounds.
- Physical properties: Electron shell arrangement influences many physical properties of elements, such as conductivity, melting point, and boiling point.
Applications and Further Exploration
The concept of electron shells and subshells extends far beyond basic atomic theory. It forms the cornerstone of:
- Molecular orbital theory: This theory builds upon the atomic orbital model to describe the bonding in molecules.
- Solid-state physics: Understanding the electron structure of solids is crucial for developing new materials with tailored properties.
- Quantum chemistry: This field uses quantum mechanics to study the electronic structure of atoms and molecules.
- Materials science: Designing and manipulating the properties of materials rely heavily on knowledge of electron arrangements.
Conclusion: A Holistic Understanding of Electron Arrangement
Electrons in the same shell share fundamental similarities in their energy levels and general location within the atom. However, the complete picture reveals nuances in their energy levels, orbital shapes, and spatial orientations due to subshells and orbitals. Understanding these similarities and differences provides a deeper appreciation for the complex world of atomic structure and its impact on the properties of matter. This knowledge is essential for advancements in various scientific and technological fields. The principles discussed here offer a framework for further exploration into the fascinating realm of quantum mechanics and its influence on the macroscopic world. The journey into the quantum world begins with comprehending these fundamental principles and opens doors to an endless expanse of scientific discovery.
Latest Posts
Latest Posts
-
Education Is Important To Society Because Quizlet
Mar 31, 2025
-
Antibodies Are Produced From Which Cells Quizlet
Mar 31, 2025
-
Ethics And Law In Leadership Edapt Quizlet
Mar 31, 2025
-
Compare And Contrast Indentured Servanthood And Slavery
Mar 31, 2025
-
Ac Theory Level 2 Lesson 8 Quizlet
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What Do Electrons In The Same Shell Have In Common . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
