What Happens To Pyruvic Acid In The Krebs Cycle
Breaking News Today
Mar 17, 2025 · 6 min read
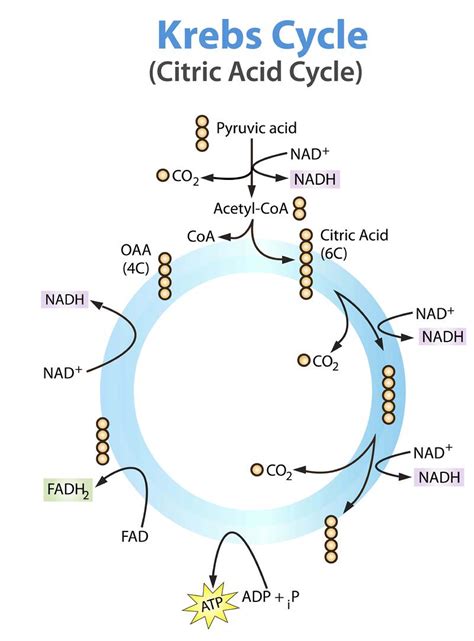
Table of Contents
What Happens to Pyruvic Acid in the Krebs Cycle? A Deep Dive into Cellular Respiration
The Krebs cycle, also known as the citric acid cycle or tricarboxylic acid (TCA) cycle, is a central metabolic pathway in all aerobic organisms. It's a crucial stage in cellular respiration, responsible for generating energy in the form of ATP (adenosine triphosphate) and reducing power in the form of NADH and FADH2. But before the Krebs cycle can even begin, pyruvic acid, the end product of glycolysis, must undergo a preparatory step. This article delves deep into the fate of pyruvic acid, exploring its transformation and the subsequent reactions within the Krebs cycle itself, highlighting the significance of each step in energy production.
From Glycolysis to the Krebs Cycle: The Pyruvate Dehydrogenase Complex
Before pyruvic acid can enter the Krebs cycle, it must first be converted into acetyl-CoA. This crucial transition occurs in the mitochondrial matrix, the inner compartment of the mitochondria, the powerhouse of the cell. The conversion is catalyzed by a large multi-enzyme complex known as the pyruvate dehydrogenase complex (PDC).
The Three Stages of Pyruvate Decarboxylation:
The PDC reaction involves three distinct stages:
-
Decarboxylation: Pyruvic acid is decarboxylated, meaning a carbon dioxide molecule is removed. This leaves behind a two-carbon fragment, an acetyl group.
-
Oxidation: The acetyl group is oxidized, meaning it loses electrons. These electrons are transferred to NAD+, reducing it to NADH. This is a crucial step in generating reducing power, essential for later ATP production in the electron transport chain.
-
Coenzyme A Attachment: Coenzyme A (CoA), a sulfur-containing molecule, binds to the acetyl group, forming acetyl-CoA. Acetyl-CoA acts as a carrier molecule, transporting the two-carbon acetyl group into the Krebs cycle.
This entire process is tightly regulated, ensuring that acetyl-CoA production matches the cell's energy demands. Inhibitors and activators of the PDC modulate its activity, maintaining a balanced energy supply. High levels of ATP and NADH inhibit PDC activity, slowing down the Krebs cycle when energy is plentiful. Conversely, low levels of ATP and NADH activate PDC, stimulating the production of acetyl-CoA and subsequently, more ATP.
The Krebs Cycle: A Circular Journey of Energy Production
With the formation of acetyl-CoA, we finally enter the Krebs cycle itself. This cycle occurs in a series of eight enzyme-catalyzed reactions within the mitochondrial matrix. Each reaction plays a critical role in generating ATP and reducing power.
Step-by-step Breakdown of the Krebs Cycle:
Let's trace the journey of acetyl-CoA through the cycle:
-
Citrate Synthesis: Acetyl-CoA (2 carbons) combines with oxaloacetate (4 carbons), a four-carbon molecule already present in the cycle, to form citrate (6 carbons). This reaction is catalyzed by citrate synthase.
-
Citrate Isomerization: Citrate is isomerized to isocitrate (6 carbons), catalyzed by aconitase. This isomerization is necessary for the next oxidative decarboxylation step.
-
Oxidative Decarboxylation 1: Isocitrate is oxidized and decarboxylated, yielding α-ketoglutarate (5 carbons), NADH, and CO2. This reaction is catalyzed by isocitrate dehydrogenase. This is another critical step in reducing power generation, providing more NADH for later use in ATP synthesis.
-
Oxidative Decarboxylation 2: α-ketoglutarate is further oxidized and decarboxylated, forming succinyl-CoA (4 carbons), NADH, and CO2. This reaction, catalyzed by α-ketoglutarate dehydrogenase, is similar to the previous oxidative decarboxylation, further contributing to the cell's reducing power.
-
Substrate-Level Phosphorylation: Succinyl-CoA is converted to succinate (4 carbons), with the release of energy used to form GTP (guanosine triphosphate), a molecule functionally equivalent to ATP. This is the only step in the Krebs cycle where ATP is directly generated through substrate-level phosphorylation.
-
Oxidation: Succinate is oxidized to fumarate (4 carbons), producing FADH2. This reaction, catalyzed by succinate dehydrogenase, is unique because it is the only Krebs cycle enzyme embedded in the inner mitochondrial membrane, directly transferring electrons to the electron transport chain.
-
Hydration: Fumarate is hydrated to form malate (4 carbons). This reaction, catalyzed by fumarase, prepares the molecule for the final oxidation step.
-
Oxidation: Malate is oxidized to regenerate oxaloacetate (4 carbons), producing NADH. This completes the cycle, ready to accept another molecule of acetyl-CoA. This reaction, catalyzed by malate dehydrogenase, replenishes the oxaloacetate required for the initial step of the cycle.
The Significance of the Krebs Cycle's Products:
The Krebs cycle isn't just a series of reactions; it's a powerhouse of energy production and metabolic precursor generation. Its primary outputs are:
-
ATP (or GTP): Direct energy currency of the cell, used to power numerous cellular processes. Though the Krebs cycle generates only a small amount of ATP directly, its contribution to the overall energy yield is significant due to the substantial production of reducing power.
-
NADH and FADH2: These reduced electron carriers are vital for oxidative phosphorylation, the final stage of cellular respiration, where the majority of ATP is produced. They transport high-energy electrons to the electron transport chain, driving proton pumping and ultimately ATP synthesis.
-
CO2: A waste product of cellular respiration, excreted from the body.
-
Metabolic Intermediates: Several intermediates of the Krebs cycle serve as precursors for the synthesis of various biomolecules, including amino acids, fatty acids, and nucleotides. This highlights the cycle's importance in anabolism (biosynthesis) as well as catabolism (energy production).
Regulation of the Krebs Cycle: A Delicate Balance
The Krebs cycle, like the pyruvate dehydrogenase complex, is highly regulated to ensure that energy production aligns with the cell's needs. Several factors influence its activity:
-
Substrate Availability: The concentration of acetyl-CoA and oxaloacetate directly affects the rate of the cycle.
-
Energy Charge: High levels of ATP and NADH inhibit several enzymes of the cycle, slowing down energy production when sufficient energy is already available.
-
Inhibitors and Activators: Specific molecules, including calcium ions (Ca2+), can modulate the activity of key Krebs cycle enzymes.
This intricate regulatory system guarantees that the cycle operates efficiently and responds dynamically to changing metabolic demands.
Beyond ATP Production: The Krebs Cycle's Multifaceted Roles
While ATP production is the primary function of the Krebs cycle, it plays a significantly broader role in cellular metabolism. Its intermediates serve as precursors for the biosynthesis of various essential molecules, including:
-
Amino acids: Several Krebs cycle intermediates are crucial in the synthesis of amino acids, the building blocks of proteins.
-
Fatty acids: Certain intermediates are diverted to fatty acid synthesis, contributing to lipid metabolism.
-
Nucleotides: Some Krebs cycle molecules contribute to the synthesis of nucleotides, the fundamental components of DNA and RNA.
This multifaceted nature underscores the Krebs cycle's importance as a central hub in cellular metabolism, linking catabolism (breakdown of molecules for energy) and anabolism (synthesis of new molecules).
Conclusion: Pyruvic Acid's Journey and the Significance of the Krebs Cycle
The transformation of pyruvic acid into acetyl-CoA and its subsequent journey through the Krebs cycle represent a pivotal stage in cellular respiration. This process, highly regulated and intricately interconnected with other metabolic pathways, generates not only ATP, the cell's primary energy source, but also crucial reducing power and building blocks for essential biomolecules. The intricate detail and multifaceted nature of the Krebs cycle highlight its fundamental importance in maintaining cellular life and supporting diverse metabolic functions. Understanding its complexities is crucial to appreciating the elegance and efficiency of cellular energy production.
Latest Posts
Latest Posts
-
Cdl Combination Test Questions And Answers Pdf
Mar 18, 2025
-
Life Insurance Exam Questions And Answers Pdf
Mar 18, 2025
-
The Direct Carry Is Used To Transfer A Patient
Mar 18, 2025
-
The Emancipation Proclamation Of January 1 1863 Quizlet
Mar 18, 2025
-
These Cards Will Get You Drunk Quizlet
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about What Happens To Pyruvic Acid In The Krebs Cycle . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
